CAR-T cell therapy includes taking T-cells from a patient & modifying them with a unique protein receptor that targets cancer cells, which is commonly used for specific blood cancers & is under investigation for other cancer types.
The process includes growing millions of these modified CAR-T cells in a lab & later on delivering them to the patient through an infusion, providing a potential weapon against the cancer. The global rise in cancer cases has driven healthcare providers to grow in favor of CAR-T cell therapy in cancer treatment.
As per the Centers for Disease Control & Prevention (CDCP) the United States, in the recent past has seen 1,752,735 new cancer cases & 599,589 cancer-related deaths, with 439 new cases & 146 deaths per 100,000 individuals. The expansion in cancer diagnoses allows healthcare providers to adopt CAR-T therapy due to its various advantages, contributing to market growth. In addition, the growing number of regulatory approvals further drives the expansion of this therapy.
However, CAR T-cell Therapy does come with some side effects, including a high reduction in antibody-producing B cells & infections, with Cytokine Release Syndrome (CRS) being a common & severe complication. CRS occurs when T cells release cytokines, which can lead to high immune responses, acting as a constraint on market growth due to safety concerns & the need for effective management of side effects.
CAR-T Cell Therapy market has experienced rapid development over recent years. CAR-T therapies are providing highly customized cancer therapies such as leukemia and lymphoma treatment with pinpoint precision; ongoing trials and innovations further highlight its promise as an innovative cancer therapy, treating an array of solid tumor types.
CAR-T therapies have seen increased adoption with growing patient demand for more effective cancer treatments, fuelled by their genetic modification to target and destroy cancerous cells directly. Their market expansion can be seen through investments into R&D as well as collaborations between biopharma companies and academic institutions.
CAR-T therapies present numerous opportunities despite hurdles such as rising treatment costs and insufficient clinical evidence in some areas, as they continue to advance rapidly. Key players have focused their efforts on increasing accessibility and efficacy for these therapies while expanding indications with approvals of CAR-T products are expected to drive market expansion further. Furthermore, advances in manufacturing supply chain logistics have enhanced production scalability; giving these therapies access to an expanded patient population.
Key Takeaways
- Market Size: The global CAR-T Cell Therapy Market is projected to reach a market value of USD 85.5 billion in 2033 from a base value of USD 7.1 billion in 2024.
- Market Definition: This market entails the use of living cells to treat or cure diseases that specialize in regenerative medicine drugs, immunotherapy, and personalized treatment processes, appreciably advancing medical outcomes and patient care.
- By Type Segment Analysis: By type, Abecma is projected to lead this market with the highest market share in 2024 & is anticipated to show subsequent growth throughout the forecasted period.
- By Application Segment Analysis: leukemia is projected to take the lead and dominate this market as it holds the majority of the market share in 2024.
- Growth Driver: Growth drivers for the CAR-T cell Therapy marketplace consist of advancements in regenerative medication, increasing incidence of chronic diseases, and huge investments in and improvement to enhance therapeutic efficacy and patient outcome.
- Regional Analysis: North America is projected to have a 56.7% share of revenue in 2024 and exert its dominance on the global cell therapy market in the upcoming years as well.
Use Cases
- Treatment of Refractory Leukemia: CAR-T cell therapy is used to treat patients with leukemia who have not responded to traditional treatments, offering a potential cure through targeted immunotherapy.
- Management of Relapsed Multiple Myeloma: For patients with multiple myeloma who have relapsed after standard treatments, CAR-T cell therapy can provide an effective option, targeting and eliminating cancerous cells.
- Targeted Therapy for Lymphoma: CAR-T cell therapy is employed in treating various forms of lymphoma, such as DLBCL and MCL, by engineering T-cells to attack specific lymphoma cells, improving patient outcomes.
- Clinical Trials for Solid Tumors: Ongoing clinical trials explore the efficacy of CAR-T cell therapy in treating solid tumors like glioblastoma and neuroblastoma, aiming to expand its application beyond hematologic cancers.
Market Dynamic
Trends
Advancements in Personalized Medicine
The global motion of CAR-T cell therapy is on the upswing for further progress with personalized medicine and continuous development. These market trends are putting forces in the industry, especially in adopting the target therapy interventions for the improvement of patients and diminishing side effects experienced by patients. Markets to watch are closely involved in the production of new CAR-T cell products since they operate in a competitive market.
Rising Collaboration between Companies and Institutions
One other emerging trend in the global CAR-T Therapy market is related to the increased level of partnership between biopharmaceutical firms the research organizations. These collaborations are mainly useful to enhance the generation of new cell Therapy based on CAR-T cells as well as to bring them to the market as quickly as possible. Such market trends may be explained by the focus on Expert Alliance business models when using industry expertise and resource potential for successful market development in this sector.
Growth Drivers
Increasing Prevalence of Cancer
The primary growth factor for the CAR-T cell therapy market is the rise in the incidence rate of cancer and more specifically blood cancer such as leukemia and lymphoma. The CAR T cell Therapy is increasing due to these diseases and the efficacy of CAR T cell Therapy hence driving the market.
Advances in Gene Editing Technologies
Technological developments in gene editing are similarly propelling the global CAR-T cell therapy market, for instance, CRISPR. These technologies allow for the better and safer alteration of T-cells which makes CAR-T cell Therapy better as well. The global market for CAR-T cell therapy is rising further due to enhancing the CAR-T cell therapy manufacturing techniques that made it easily and reasonably available.
Growth Opportunities
Expansion in North America
Factors such as the high incidence of cancer and well-developed healthcare facilities are making the North American CAR-T cell therapy market highly prospective. The cast players are focusing their efforts on increasing the size of the therapy business within this region. Also, an appropriate number of cancer research grants and an appropriate government support system for cancer clinical trials in the region are expected to collectively propel the market growth in this region during the given time period.
Emerging Markets in Asia-Pacific
The growth rate of emerging markets that are still popular in Asia-Pacific is very high and, as such, can be seen to hold a lot more potential in the CAR-T cell therapy market. Increasing consciousness of people about the details of different types of cancer treatments and the availability of better healthcare services are major factors that enhance the need for CAR-T cell therapy in these regions. global market study shows that the Asia-Pacific CAR-T
cell therapy market will grow at a fast pace in the coming years offering the player an opportunity to tap a global market.
Restraints
High Cost of Therapy
Another key challenge in the CAR-T cell therapy business is the price at which these Therapies are conducted. Currently, CAR-T cell therapy is rather costly and this is a huge barrier to the implementation of this form of treatment for a larger population, especially in LMIC. This remains a bottleneck that slows the pace of the market’s development and becomes a problem for the players in the CAR-T cell therapy industry to reduce the cost of these treatments.
Potential for Severe Side Effects
One more limitation is connected with the side effects and complications of CAR-T cell therapy. These Therapies have the risk of inducing cytokine release syndrome (CRS) and neurotoxicity for which appropriate precautions and measures need to be taken and constant monitoring should be done. The consequences and possible complications can dissuade the patients and healthcare providers from availing and performing CAR-T cell therapy thus having an overall effect on the therapy market size and growth.
Research Scope and Analysis
By Type
Abecma is a major type of CAR-T cell therapy that drives the growth of the market, as it is developed and designed for the treatment of numerous myeloma, a challenging form of blood cancer. Abecma works by modifying a patient's own T cells to focus on a specific protein found in myeloma cells, which helps the immune system recognize & attack the cancer. This therapy has gained significant attention owing to its success in clinical trials, demonstrating a promising ability to induce deep & lasting responses in some patients.
While there can be side effects, Abecma offers hope for those who have exhausted other treatment options for multiple myeloma. Its emergence enhances the potential of CAR-T cell Therapy to revolutionize the treatment landscape for various types of cancer. Continued research and developments in this field are eagerly anticipated to expand its application and effectiveness further.
By Application
In terms of application, the market is segmented into leukemia, multiple myeloma, lymphoma, autoimmune disorders, and others, of which leukemia is projected to hold the major share as a segment in 2024, and is expected to keep the lead throughout the forecasted period. CAR-T cell therapy has emerged as a new approach to the treatment of leukemia. In addition, it has shown high success in addressing acute lymphoblastic leukemia & few types of chronic lymphocytic leukemia.
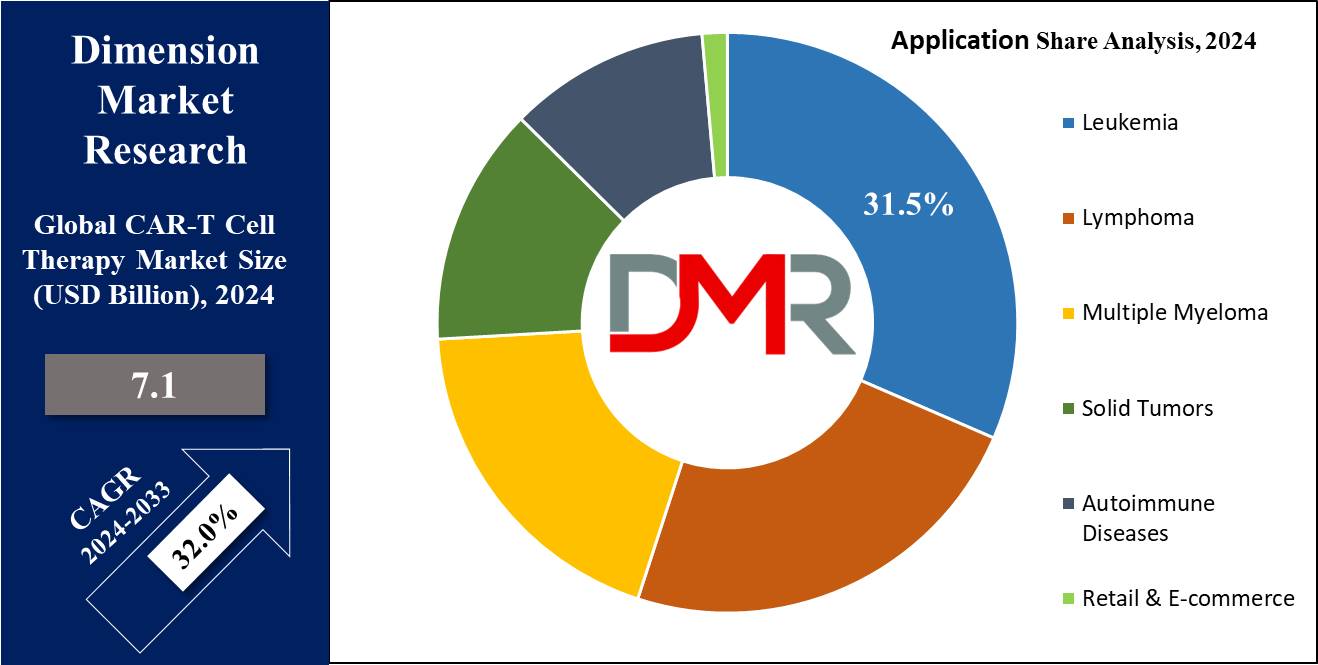
In addition, a patient's own T cells are genetically modified to target leukemia cells more effectively. , which are then injected back into the patient's body, where they recognize & attack cancer cells with greater accuracy, and also offer hope to patients who may not have responded to traditional Therapy and have demonstrated high suspension rates. While it comes with some side effects, CAR-T therapy's potential to give long-term remission for leukemia patients marks a significant advancement in cancer treatment. As research continues, this application holds promise for broader leukemia management.
By End User
Hospitals are a major driving factor for the global CAR-T cell Therapy market and are anticipated to grow significantly over the forecasted period, as they play a major role in CAR-T cell therapy. These advanced Therapy, designed to treat certain types of cancer, require advanced infrastructure & highly trained medical teams for their administration & patient care. Hospitals serve as the primary centers for CAR-T cell therapy delivery, providing a controlled environment for these intricate treatments.
They contain all the necessary facilities for cell collection, genetic modification, & reinfusion, ensuring patients receive the therapy safely. Moreover, hospitals are responsible for monitoring patients during & after treatment, addressing potential side effects, and providing critical support in case of adverse reactions. As CAR-T Therapy continues to advance, hospitals remain central to the accessibility and success of these groundbreaking treatments.
The CAR-T Cell Therapy Market Report is segmented on the basis of the following
By Type
- Abecma Abecma (idecabtagene vicleucel)
- Kymriah (tisagenlecleucel)
- Breyanzi (lisocabtagene maraleucel)
- Tecartus (brexucabtagene autoleucel)
- Yescarta (axicabtagene ciloleucel)
- Others
By Target Antigen
- CD19
- BCMA (B-cell maturation antigen)
- CD20
- CD22
- Dual Targets (CD19/CD22, CD19/BCMA, etc.)
- Others
By Application
- Leukemia
- Acute Lymphocytic Leukemia (ALL)
- Chronic Lymphocytic Leukemia (CLL)
- Acute Myeloid Leukemia (AML)
- Lymphoma
- Diffuse Large B-cell lymphoma (DLBCL)
- Mantle Cell Lymphoma (MCL)
- Follicular Lymphoma (FL)
- Primary Mediastinal Large B-cell lymphoma (PMBCL)
- High-grade B-cell lymphoma (HGBL)
- Others
- Multiple Myeloma (MM)
- Solid Tumors
- Glioblastoma
- Neuroblastoma
- Others
- Autoimmune Diseases
- Others
By End User
- Hospitals
- Academic and Research Hospitals
- General Hospitals
- Specialized Cancer Hospitals
- Cancer Care Treatment Centers
- Clinical Research Organizations (CROs)
- Biotechnology and Pharmaceutical Companies
- Others
Regional Analysis
North America is projected to lead the CAR-T cell therapy market
with a significant 56.7% share of the revenue in 2024, which is primarily due to the strong research infrastructure, commercial activities, & a large number of clinical studies conducted on T-cell Therapy across the United States. In addition, the region has experienced a growth in regulatory approvals in both the US & Canada, alongside changes in reimbursement policies, which have driven the growth of adoption of this Therapy & promoted substantial market growth.
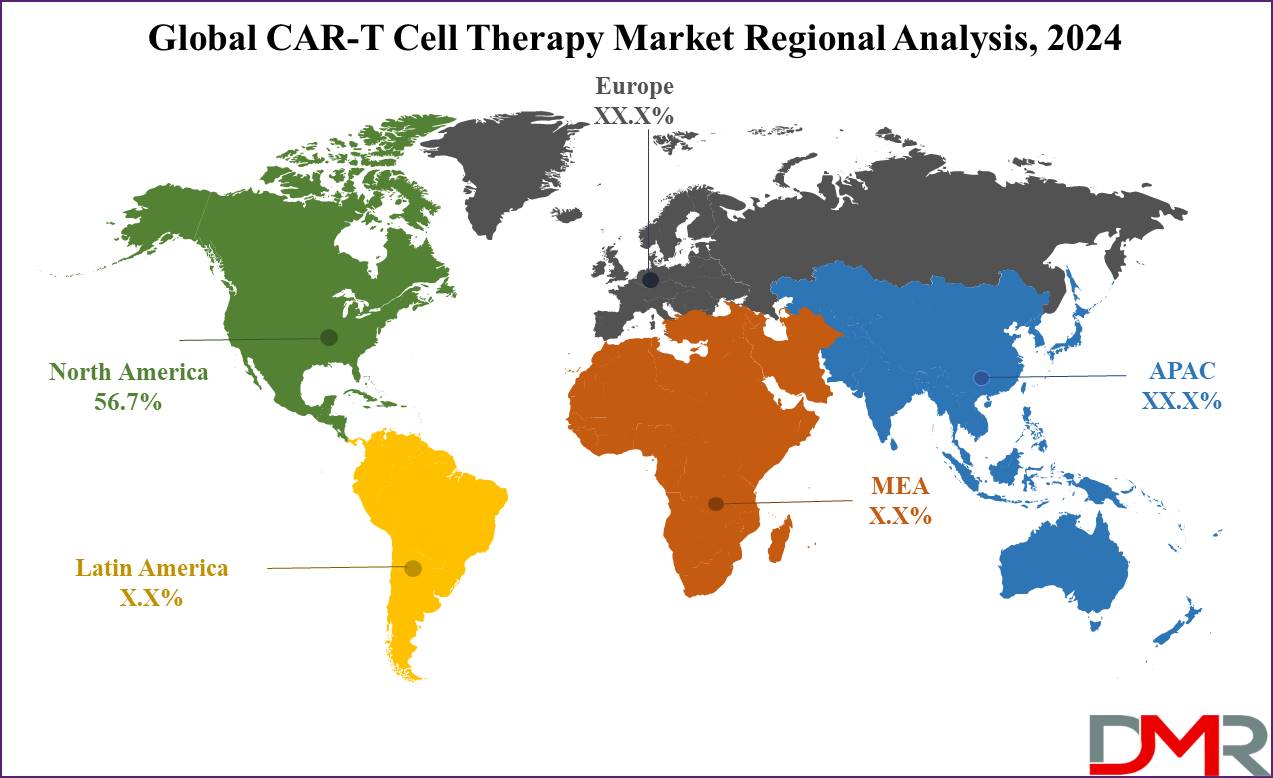
Moreover, the Asia Pacific region is anticipated for rapid expansion in the coming years. China, in particular, stands out as a major market for CAR-T Therapy. The nation has taken the lead in hosting the most registered clinical trials for these Therapy, making it an important hub for CAR-T developments in recent years, which is a result of concerted efforts through government investments & healthcare reforms, contributing to the region's fastest growth in the CAR-T cell therapy market.
By Region
North America
Europe
- Germany
- The U.K.
- France
- Italy
- Russia
- Spain
- Benelux
- Nordic
- Rest of Europe
Asia-Pacific
- China
- Japan
- South Korea
- India
- ANZ
- ASEAN
- Rest of Asia-Pacific
Latin America
- Brazil
- Mexico
- Argentina
- Colombia
- Rest of Latin America
Middle East & Africa
- Saudi Arabia
- UAE
- South Africa
- Israel
- Egypt
- Rest of MEA
Competitive Landscape
The CAR-T cell therapy market experiences moderate competition, featuring numerous global players who are employing various strategies to strengthen their position in the market, including looking for partnerships or collaborations, launching new products, expanding into different regions, & merging with or acquiring other businesses. This dynamic approach is helping them grow and make their mark in the industry.
For instance, in June 2022, the FDA granted its approval to Bristol Myers Squibb for a CAR-T cell therapy called Breyanzi (lisocabtagene maraleucel), which is particularly developed to treat adult patients with a type of cancer known as large B-cell lymphoma (LBCL). This approval signifies that Breyanzi has met the necessary requirements and is now recognized as a safe & effective treatment for this condition.
Some of the prominent players in the Global CAR-T Cell Therapy Market are
- Johnson & Johnson
- Novartis AG
- Eli Lilly & Company
- Celyad Oncology
- Bristol-Myers Squibb Company
- ACRO Biosystems
- Servier Laboratories
- Miltenyi Biotec
- Gilead Sciences Inc
- Sorrento Therapeutics Inc
- Other Key Players
Recent Developments
- In November 2023, Selecta Biosciences, Inc. announced that it merged with Cartesian Therapeutics, Inc. With the cash from both companies at closing and the proceeds of the concurrent private financing, the combined company is expected to have over $110 million on hand to support the development of the Cartesian pipeline through the Phase 3 study of lead product candidate, Descartes-08, a potential first-in-class RNA-engineered chimeric antigen receptor T-cell therapy (rCAR-T) for the treatment of MG, as well as the advancement of additional RNA cell therapy programs.
- In December 2023, Bristol-Myers Squibb announced that it received manufacturing and marketing approval of the supplemental New Drug Application for an additional indication for Abecma (idecabtagene violence), a B-cell maturation antigen (BCMA)-directed chimeric antigen receptor (CAR) T cell immunotherapy, for patients with relapsed or refractory multiple myeloma (RRMM) who have received at least two prior Therapy, including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 antibody.
- In September 2023, 2seventy Bio, Inc. and JW Therapeutics announced their intention to expand their strategic alliance. The companies intend to add up to two additional candidates from the 2seventy portfolio, one in solid tumor indications using T-cell receptor (TCR) based technology and a second in autoimmune disease using a CAR T cell approach.
- In May 2023, Autolus Therapeutics plc announced that the abstract for the pivotal Phase 2 FELIX study of obecabtagene autoleucel (obe-cel) in relapsed/refractory (r/r) adult B-cell Acute Lymphoblastic Leukemia (ALL) has been selected for an oral presentation at the European Hematology Association (EHA) 2023 Congress.
- In June 2022, Bristol Myers Squibb received FDA approval for Breyanzi (lisocabtagene maraleucel), a CD19-directed chimeric antigen receptor (CAR) T-cell therapy, for the treatment of adult patients with large B-cell lymphoma (LBCL).
- In April 2022, Autolus Therapeutics plc announced that it has collaborated with Cardinal Health Inc. to support the launch and commercialization of its CAR T-cell Therapy in the U.S., subject to FDA approval.
- In April 2022, Kite, a Gilead Company, received FDA approval for Yescarta (axicabtagene ciloleucel) CAR T-cell therapy for adult patients with large B-cell lymphoma that is refractory to first-line chemoimmunotherapy or that relapses within 12 months of first-line chemoimmunotherapy.
Report Details
| Report Characteristics |
| Market Size (2024) |
USD 7.1 Bn |
| Forecast Value (2033) |
USD 85.8 Bn |
| CAGR (2024–2033) |
32.0% |
| Historical Data |
2018 – 2023 |
| Forecast Data |
2024 – 2033 |
| Base Year |
2023 |
| Estimate Year |
2024 |
| Report Coverage |
Market Revenue Estimation, Market Dynamics, Competitive Landscape, Growth Factors and etc. |
| Segments Covered |
By Type (Abecma, Kymriah, Breyanzi, Tecartus, Yescarta, and Others), By Target Antigen (CD19, BCMA, CD20, CD22, Dual Targets, Others), By Application (Leukemia, Lymphoma, Multiple Myeloma, Solid Tumors, Autoimmune Disorders, and Others), By End User (Hospitals, Cancer Care Treatment Centers, Clinical Research Organizations (CROs), Biotechnology and Pharmaceutical Companies and Others) |
| Regional Coverage |
North America – The US and Canada; Europe – Germany, The UK, France, Russia, Spain, Italy, Benelux, Nordic, & Rest of Europe; Asia-Pacific – China, Japan, South Korea, India, ANZ, ASEAN, Rest of APAC; Latin America – Brazil, Mexico, Argentina, Colombia, Rest of Latin America; Middle East & Africa – Saudi Arabia, UAE, South Africa, Turkey, Egypt, Israel, & Rest of MEA |
| Prominent Players |
Johnson & Johnson, Novartis AG, Eli Lilly & Company, Celyad Oncology, Bristol-Myers Squibb Company, ACRO Biosystems, Servier Laboratories, Miltenyi Biotec, Gilead Sciences Inc, Sorrento Therapeutics Inc, and Other Key Players |
| Purchase Options |
HVMN Inc., Thync Global Inc., Apple Inc., Fitbit Inc., TrackmyStack, OsteoStrong, The ODIN, Thriveport LLC, Muse, Moodmetric, and Other Key Players |