Market Overview
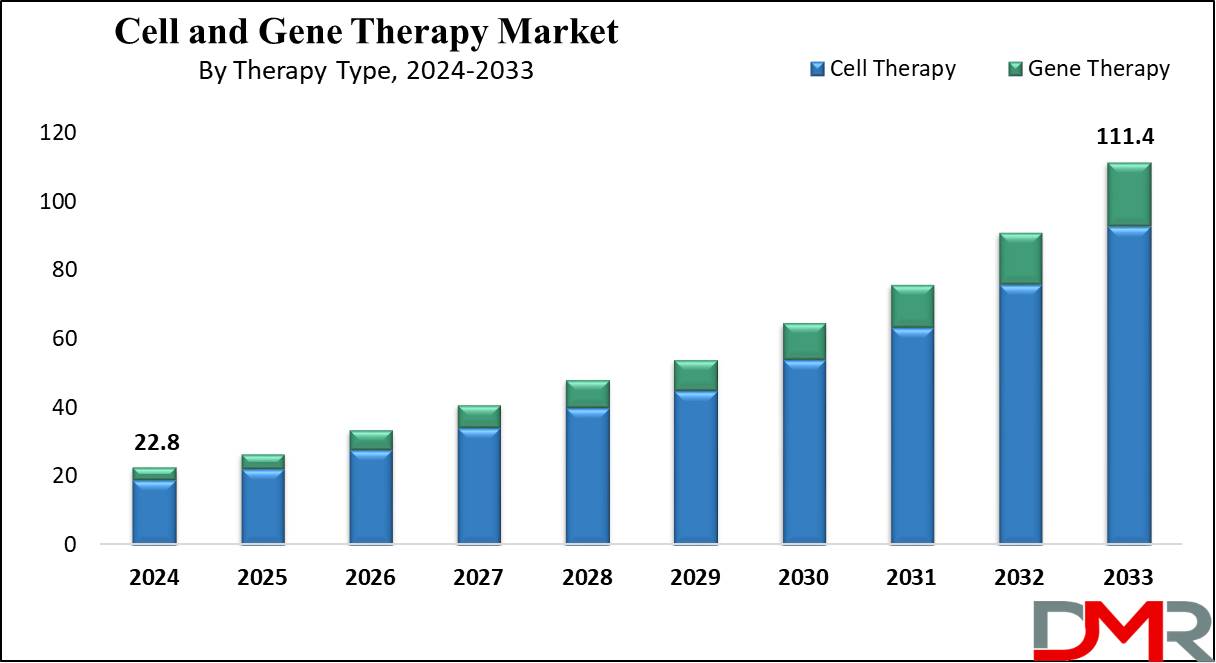
The Global Cell and Gene Therapy Market is projected to reach USD 22.8 billion in 2024 and grow at a compound annual growth rate of 19.3% from there until 2033 to reach a value of USD 111.4 billion.
Cell and gene therapy provides solutions related to genes and cells, as it deals with research & development, testing, production, and distribution of products and treatment procedures related to genes and cells. Hospitals, research laboratories, pharmaceutical companies, pharmacies, research institutions, and universities are included in delivering the applications associated with gene & cell therapies, supported by ongoing genomic medicine advancements across the healthcare sector.
Gene and cell therapies are developed to secure, treat, or potentially cure various diseases. This includes advanced forms of Cell Therapy, including Stem Cell Therapy, which are revolutionizing how chronic conditions and degenerative diseases are managed. The potential of these therapies to cure, treat, or prevent diseases that are life-threatening increases the demand and boosts the growth of the market, especially with the rise of regenerative therapeutics and next-generation advanced biologics.
The US Cell and Gene Therapy Market
The
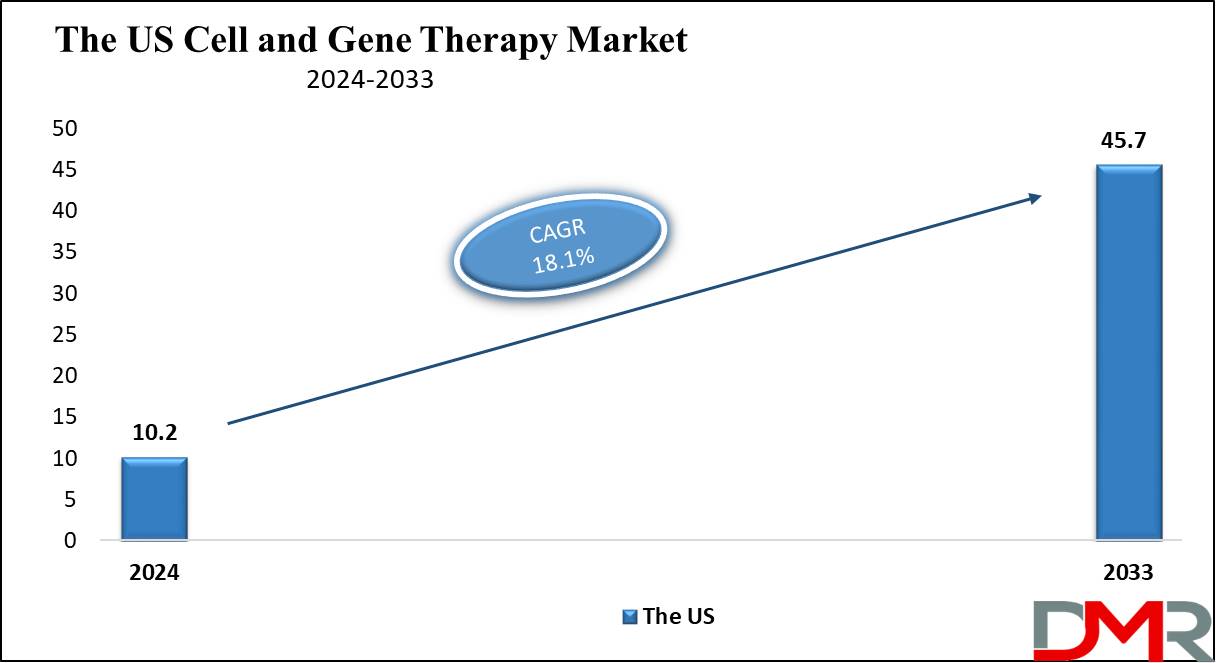
US Cell and Gene Therapy Market is projected to reach
USD 10.2 billion in 2024 at a compound annual
growth rate of 18.1% over its forecast period.
The US provides numerous growth opportunities in the cell and gene therapy market through its strong research infrastructure, a high number of clinical trials, and favorable regulatory assistance from the FDA. Large investments, partnerships, and development in manufacturing technologies further drive market expansion. In addition, the growth in demand for customized medicine and therapies targeting cancer and genetic disorders fuels growth opportunities.
Further, a strong research ecosystem, with significant clinical trials and the FDA helps accelerate innovation. However, high development & treatment costs remain major restraints, limiting patient access & insurance coverage. Overcoming manufacturing challenges & ensuring affordable therapies, especially in complex
Stem Cell Therapy procedures, are vital to unlocking the market's full potential.
Key Takeaways
- Market Growth: The Cell and Gene Therapy Market size is expected to grow by 84.7 billion, at a CAGR of 19.3% during the forecasted period of 2025 to 2033.
- By Therapy Type: The Cell Therapy segment is anticipated to get the majority share of the Cell and Gene Therapy Market in 2024.
- By Indication: The Oncological disorders segment is expected to be leading the market in 2024
- By End User: The hospital segment is expected to get the largest revenue share in 2024 in the Cell and Gene Therapy Market.
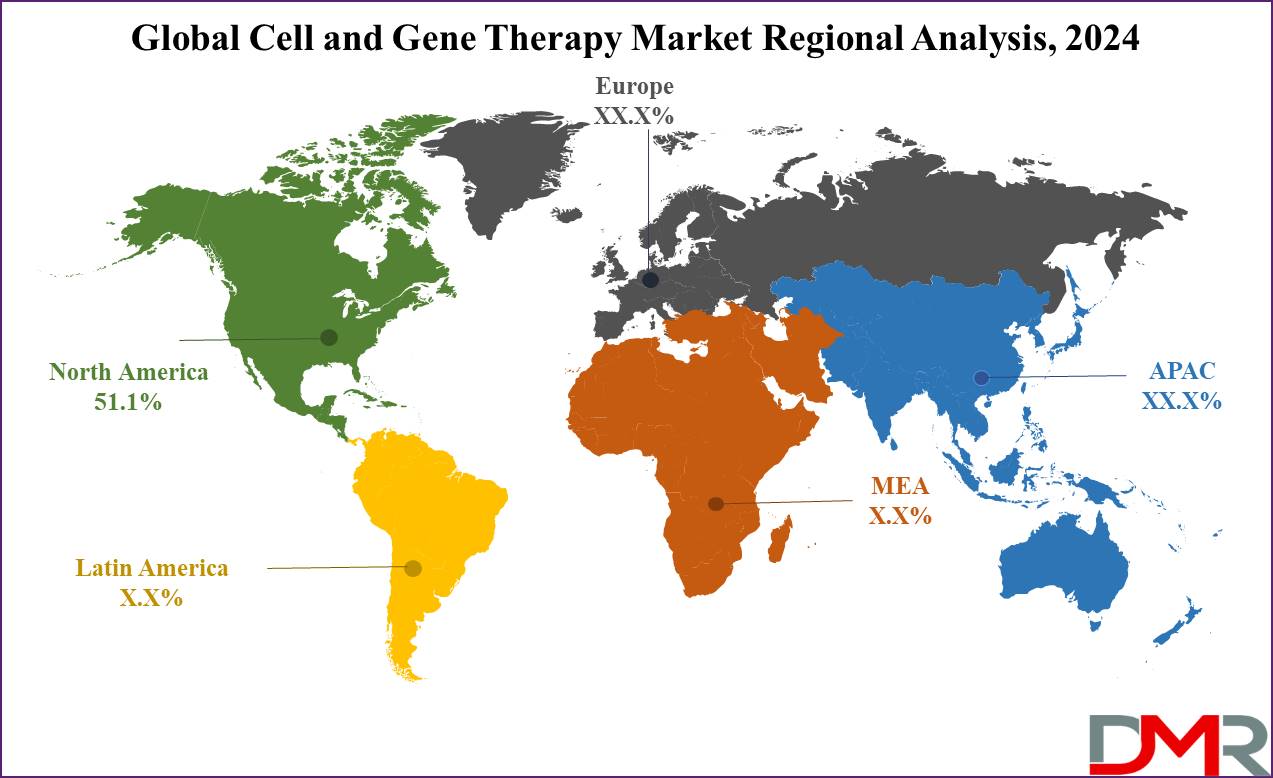
- Regional Insight: North America is expected to hold a 51.1% share of revenue in the Global Cell and Gene Therapy Market in 2024.
- Use Cases: Some of the use cases of Cell and Gene Therapy include regenerative medicine, rare genetic disorders, and more.
Use Cases
- Cancer Treatment (CAR-T Therapy): CAR-T therapy develops patient T-cells to find and remove cancer cells. It has been remarkable in treating leukemia and lymphoma, providing a customized and potentially curative approach.
- Rare Genetic Disorders: Gene therapy addresses inherited diseases by delivering functional genes, like spinal muscular atrophy (SMA) and hemophilia. It attacks the root cause by correcting defective genes at the molecular level.
- Regenerative Medicine: Cell therapies, like stem cell transplantation, develop damaged tissues and organs. They have applications such as repairing heart tissue after myocardial infarctions and regenerating neurons in neurodegenerative diseases.
- Immunological Disorders: Gene-modified immune cells can fight autoimmune diseases or viral infections, which improves immune response, exemplified by therapies being designed for HIV and severe combined immunodeficiency (SCID).
Market Dynamic
Driving Factors
Technological Advancements and Innovations
Breakthroughs in gene-editing technologies (e.g., CRISPR) and development in viral/non-viral delivery systems are accelerating the expansion of safer, more effective therapies, driving market growth.
Rising Prevalence of Chronic and Genetic Diseases
The expanding incidence of cancer, rare genetic disorders, and age-related conditions creates a need for innovative treatment options, driving the adoption of cell and gene therapies globally.
Restraints
High Development and Treatment Costs
The complex manufacturing processes and regulatory needs lead to high R&D costs. Treatments like CAR-T therapies are expensive, limiting accessibility & reimbursement options.
Regulatory and Logistical Challenges
Navigating strict regulatory approvals and ensuring smooth supply chains creates major hurdles, delaying market expansion and commercialization efforts.
Opportunities
Expansion into Emerging Markets
The increased healthcare infrastructure and government initiatives in regions like Asia-Pacific provide new opportunities for market players to expand clinical trials and commercialization efforts.
Advancements in Personalized Medicine
The growing focus on customized therapies opens doors for targeted treatments, like patient-specific CAR-T therapies, allowing more effective solutions and fostering market growth.
Trends
Increased Collaboration and Partnerships
Pharma companies are highly collaborating with biotech firms & research institutions to expand the development of cell and gene therapies. These partnerships focus on combining expertise in clinical trials, manufacturing, and regulatory approvals to bring therapies to market faster.
Adoption of Allogeneic "Off-the-Shelf" Therapies
Allogeneic cell therapies, derived from donor cells, are gaining traction as they provide the potential for "off-the-shelf" solutions, minimizing wait times and costs in comparison to autologous therapies, which is driving innovation in scalable, ready-to-use treatment options.
Research Scope and Analysis
By Therapy Type
The global cell and gene therapy market is broadly divided into two key segments: cell therapy and gene therapy. In 2024, the cell therapy segment is projected to account for the largest share of market revenue, driven by its extensive potential in treating autoimmune diseases, various cancers, and urinary disorders.
This growth is further supported by substantial funding directed toward the development of new cell lines and by companies adopting both organic and inorganic strategies to expand and enhance their cell therapy manufacturing capabilities. Additionally, adjacent sectors such as organic pigments, which play a role in bioprocessing materials and labeling technologies, are also experiencing heightened demand as innovation in advanced therapeutics accelerates.
Expanding the growth of the market, mainly including the introduction of healthy, modified cells into the body to replace diseased or damaged ones, focusing on improving the patient's overall health by restoring normal cellular function or eliminating the disease. Further, the gene therapy segment is projected to maintain the second-largest market share over the forecast period while growing at a significant rate.
Gene therapy aims to alter the genetic makeup of cells to treat or even reverse diseases by repairing damaged genetic material, which focuses on addressing the root causes of genetic disorders, providing potential cures rather than just symptom management. As research and development in this field continue to advance, gene therapy remains a vital area within the broader landscape of cell and gene therapies, promising transformative solutions for various health conditions.
By Indication
The global cell and gene therapy market based on indications is categorized into genetic disorders, cardiovascular disorders, neurological disorders, oncological disorders, and others. Among these, the oncological disorders segment is set to capture the largest share of market revenue in 2024, which is driven by major R&D in cell and gene therapy, which has the potential to address the root causes of many hereditary disorders and effectively manage diseases.
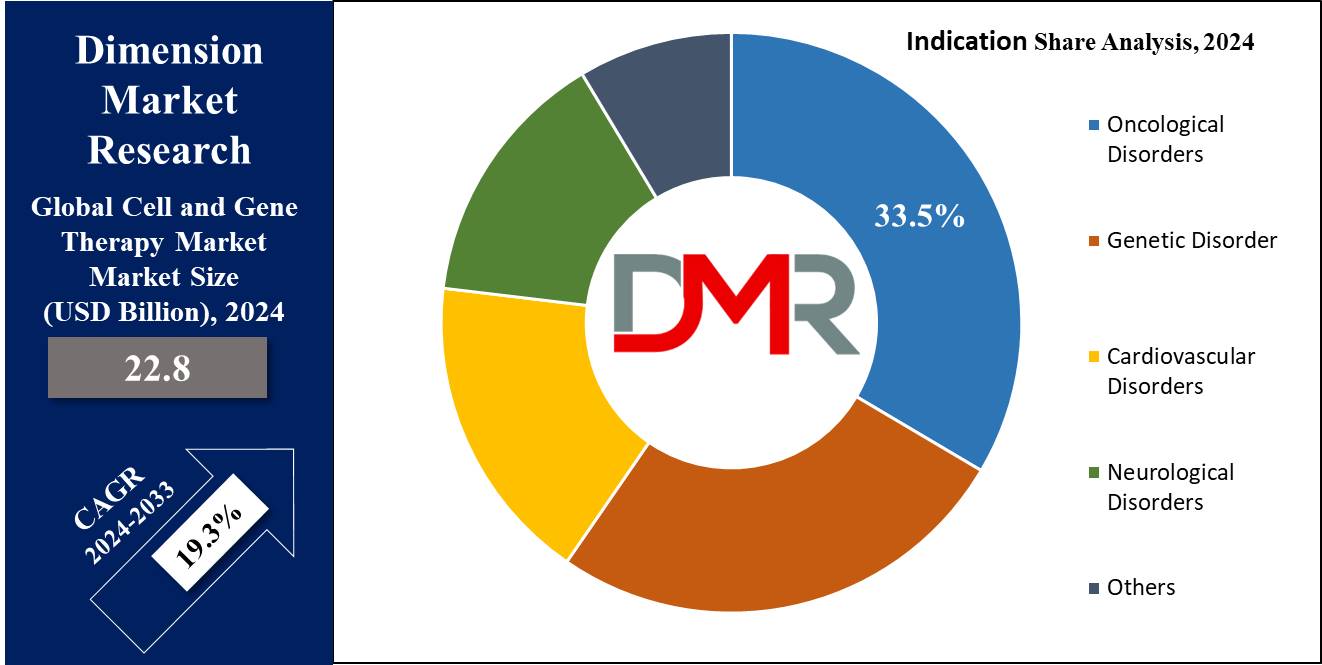
The development of innovative treatments in the field of oncology is mainly notable, as these therapies focus on targeting cancer at a genetic level, providing hope for more effective and customized treatment options. Further, the genetic disorder segment is anticipated to experience the fastest growth rate throughout the forecast period, which can be due to the growth in genetic and chronic diseases, along with various government initiatives to promote public awareness about genetic testing and diagnosis.
As researchers look into the genetic foundations of cardiovascular diseases, they are creating new methods for looking, analyzing, and treating patients with heart & vascular conditions. These developments not only improve patient outcomes but also accelerate the potential applications of cell & gene therapies, developing a more advanced market for innovative treatments targeting genetic disorders.
By Delivery Method
The cell and gene therapy market is categorized by delivery mode into two types, which are in vivo and ex vivo therapies. The in vivo therapy segment is expected to experience significant growth during the forecast period and is anticipated to lead in 2024. In gene therapy, apart from cell therapies, two primary approaches are used: ex vivo and in vivo. For gene therapy to be effective, modified human genes must be introduced into the cells of the infected individual.
In in vivo therapy, genetic material is provided directly to the targeted cells, like cancer cells, while they remain in the patient’s body. Further, in ex vivo therapy, cells are first extracted from the patient, treated with genetic transformation outside the body, and then reintroduced into the patient, which allows for careful alteration of the genes before the cells are returned, ensuring a more stable approach. Both methods focus on correcting genetic defects or enhancing cellular functions to treat many diseases, showcasing innovative strategies within the cell and gene therapy landscape.
By End User
The global cell and gene therapy market is segmented by end-users into hospitals, cancer care centers, academic and research institutions, and other facilities. Among these hospitals, they are expected to exhibit the highest growth along with the majority share throughout the forecast period, which can be owing to hospitals' ability to provide access to advanced therapies, allowing patients to get innovative treatments that may not be available elsewhere.
In addition, cancer care centers are also set to experience significant growth within the cell and gene therapy markets. These specialized centers are at the top of research & treatment for cancer, often leading in the implementation of advanced therapies customized to battle various types of cancer. By looking at personalized treatment plans and using the latest technological developments, cancer care centers attract patients looking for specialized care.
The Cell and Gene Therapy Market Report is segmented on the basis of the following
By Therapy Type
- Cell Therapy
- Stem Cells
- T Cells
- Dendritic Cells
- NK Cells
- Tumor Cells
- Gene Therapy
By Indication
- Genetic Disorders
- Cardiovascular Disorders
- Neurological Disorders
- Oncological Disorders
- Others
By Delivery Method
By End User
- Hospitals
- Academic & Research Centers
- Cancer Care Centers
- Others
Regional Analysis
The North American region is anticipated to hold a major share of the cell and gene therapy market, accounting for
51.1% of revenue in 2024, which leads the way with the highest number of gene therapy clinical trials, supporting over 400 companies actively engaged in developing cell and gene therapy products for many medical conditions.
The growth in North America is largely driven by developments in healthcare infrastructure, a major number of ongoing clinical trials, and better involvement from firms in gene and cell therapy development. The favorable regulatory landscape, mainly in the U.S., has played a vital role, with the FDA implementing a collaborative regulatory approach that provides direct & consistent support for these innovative therapies.
Further, in Europe, various new collaborative research & innovation initiatives have emerged under the Horizon 2021 program, aiming at gene therapy using viral vectors. These initiatives are anticipated to boost the growth of cell and gene therapy manufacturing services throughout Europe, which will be further assisted by the region's developed infrastructure and a skilled workforce, which are important for developing research & clinical applications.
As European countries invest in these initiatives and enhance their capabilities, the potential for major developments in cell and gene therapies will increase, improving their global competitiveness in this rapidly evolving market.
By Region
North America
Europe
- Germany
- The U.K.
- France
- Italy
- Russia
- Spain
- Benelux
- Nordic
- Rest of Europe
Asia-Pacific
- China
- Japan
- South Korea
- India
- ANZ
- ASEAN
- Rest of Asia-Pacific
Latin America
- Brazil
- Mexico
- Argentina
- Colombia
- Rest of Latin America
Middle East & Africa
- Saudi Arabia
- UAE
- South Africa
- Israel
- Egypt
- Rest of MEA
Competitive Landscape
The competitive landscape of the Cell and Gene Therapy market is rapidly transforming, with both established pharmaceutical players and emerging biotech companies driving innovation. The market is shaped by strong research efforts, strategic collaborations, and the aim of developing new therapies for cancer, genetic disorders, and rare diseases. Companies compete on factors like technological expertise, clinical trial success, regulatory approvals, and manufacturing capabilities.
There is a major focus on expanding treatment pipelines, enhancing delivery systems, and addressing scaling challenges. In addition, organizations are investing highly in partnerships with academic institutions to access advanced research, while regional expansion into emerging markets provides further opportunities for competitive advantage.
Some of the prominent players in the Global Cell and Gene Therapy are
- Amgen
- Novartis
- Pfizer
- Biogen
- Bristol-Myers Squibb Company
- Thermo Fisher Scientific
- Kolon TissueGene Inc
- JCR Pharmaceuticals
- Alnylam Pharmaceuticals
- Sanofi
- Other Key Players
Recent Developments
- In April 2024, The President of India launched India’s first home-grown gene therapy for cancer at IIT Bombay, which is a major breakthrough in the battle against cancer. As this line of treatment, named “CAR-T cell therapy," is accessible and less expensive, it provides new hope for the whole of humankind. Further, CAR-T cell therapy is considered to be one of the most exceptional developments in medical science and has been available in developed nations for some time.
- In February 2024, BioNTech SE along with Autolus Therapeutics plc introduced a strategic collaboration focused on developing both companies’ autologous CAR-T programs towards commercialization, pending regulatory authorizations. In connection with the strategic collaboration, the companies entered into a license and option agreement and a securities purchase agreement.
- In January 2024, AbbVie and Umoja Biopharma introduced two exclusive options and license agreements to develop multiple in-situ generated CAR-T cell therapy candidates in oncology utilizing Umoja's proprietary VivoVecTM platform, as the first agreement provides AbbVie an exclusive option to license Umoja's CD19 directed in-situ generated CAR-T cell therapy candidates, which includes UB-VV111, Umoja's lead clinical program for hematologic malignancies currently at the IND-enabling phase.
- In December 2023, The US FDA approved two major treatments, Casgevy and Lyfgenia, showing the first cell-based gene therapies for the treatment of sickle cell disease (SCD) in patients 12 years and older. In addition, one of these therapies, Casgevy, is the first FDA-approved treatment to use a type of new genome editing technology, signaling an innovative advancement in the field of gene therapy.
- In November 2023, AstraZeneca unveiled a collaboration and investment agreement with Cellectis, a clinical-stage biotechnology company, to expand the development of next-generation therapeutics in areas of high unmet need, like immunology, oncology, and rare diseases. Further, in the collaboration, AstraZeneca will use the Cellectis proprietary gene editing technologies &manufacturing capabilities, to develop new cell and gene therapy products, enhancing AstraZeneca’s growing product portfolio in this space.
Report Details
|
Report Characteristics
|
| Market Size (2024) |
USD 22.8 Bn |
| Forecast Value (2033) |
USD 111.5 Bn |
| CAGR (2024-2033) |
19.3% |
| Historical Data |
2018 – 2023 |
| The US Market Size (2024) |
USD 10.2 Bn |
| Forecast Data |
2025 – 2033 |
| Base Year |
2023 |
| Estimate Year |
2024 |
| Report Coverage |
Market Revenue Estimation, Market Dynamics, Competitive Landscape, Growth Factors and etc. |
| Segments Covered |
By Therapy Type (Cell Therapy and Gene Therapy), By Indication (Genetic Disorders, Cardiovascular Disorders, Neurological Disorders, Oncological Disorders, and Others), By Delivery Method (In Vivo and Ex Vivo), By End User (Hospitals, Academic & Research Centers, Cancer Care Centers, and Others) |
| Regional Coverage |
North America – The US and Canada; Europe – Germany, The UK, France, Russia, Spain, Italy, Benelux, Nordic, & Rest of Europe; Asia- Pacific– China, Japan, South Korea, India, ANZ, ASEAN, Rest of APAC; Latin America – Brazil, Mexico, Argentina, Colombia, Rest of Latin America; Middle East & Africa – Saudi Arabia, UAE, South Africa, Turkey, Egypt, Israel, & Rest of MEA |
| Prominent Players |
Amgen, Novartis, Pfizer, Biogen, Bristol-Myers Squibb Company, Thermo Fisher Scientific, Kolon TissueGene Inc, JCR Pharmaceuticals, Alnylam Pharmaceuticals, Sanofi, and Other Key Players |
| Purchase Options |
We have three licenses to opt for: Single User License (Limited to 1 user), Multi-User License (Up to 5 Users) and Corporate Use License (Unlimited User) along with free report customization equivalent to 0 analyst working days, 3 analysts working days and 5 analysts working days respectively. |
Frequently Asked Questions
The Global Cell and Gene Therapy Market size is expected to reach a value of USD 22.8 billion in 2024 and is expected to reach USD 111.4 billion by the end of 2033.
North America is expected to have the largest market share in the Global Cell and Gene Therapy Market with a share of about 51.1% in 2024.
The Cell and Gene Therapy Market in the US is expected to reach USD 10.2 billion in 2024.
Some of the major key players in the Global Cell and Gene Therapy Market are Amgen, Novartis, Pfizer, and others.
The market is growing at a CAGR of 19.3 percent over the forecasted period.