Market Overview
The Global Deep Brain Stimulation (DBS) Devices Market is projected to reach USD 3.9 billion in 2025 and is expected to expand at a compound annual growth rate (CAGR) of 10.4% from 2025 to 2034, attaining a market value of approximately USD 9.4 billion by 2034. Market growth is primarily driven by the rising prevalence of neurological disorders such as Parkinson’s disease, essential tremor, dystonia, epilepsy, and obsessive-compulsive disorder (OCD). Advancements in neurostimulation technology, including directional leads, closed-loop systems, and AI-enabled programming algorithms, are enhancing therapeutic precision and patient outcomes.
Furthermore, increasing adoption of minimally invasive neurosurgical procedures, growing investments in brain-computer interface research, and expanding availability of MRI-compatible and rechargeable DBS systems are fueling market expansion. The surge in geriatric populations, improved reimbursement frameworks, and the growing use of DBS for psychiatric and movement disorders further reinforce the long-term growth outlook of the global market.

The global landscape for Deep Brain Stimulation (DBS) devices is characterized by a trajectory of robust expansion, fueled by the escalating prevalence of neurological disorders and continuous technological refinement. A prominent trend involves the development of directional leads and current-steering technology, which allows for more precise targeting of neural structures. This enhanced precision mitigates side effects and expands the therapeutic window for conditions like Parkinson's disease and essential tremor.
Furthermore, the integration of advanced imaging techniques, such as high-field MRI, with sophisticated surgical planning software is revolutionizing procedural accuracy, leading to improved patient outcomes and heightened clinician confidence in adopting this neuromodulation therapy.
Significant opportunities for market proliferation lie in the exploration of new neurological and psychiatric indications. Clinical trials are actively investigating the efficacy of DBS for conditions such as treatment-resistant depression, obsessive-compulsive disorder, and epilepsy. Success in these areas would substantially broaden the addressable patient population beyond traditional movement disorders. Concurrently, the miniaturization of device components and the advent of rechargeable implantable pulse generators are addressing key patient concerns regarding convenience and longevity, making the therapy more appealing and manageable over the long term.
Despite the optimistic outlook, the market faces considerable restraints, primarily the substantial cost associated with the DBS procedure and the devices themselves. This high economic barrier can limit access, particularly in developing healthcare systems and for patients without comprehensive insurance coverage.
Moreover, the inherent risks of neurosurgical intervention, including intracranial hemorrhage and infection, alongside the potential for hardware-related complications, present significant challenges. Stringent and lengthy regulatory approval processes for new devices and indications also act as a brake on the pace of innovation and market entry for new competitors.

The US Deep Brain Stimulation Devices Market
The US Deep Brain Stimulation Devices Market is projected to reach USD 1.2 billion in 2025 at a compound annual growth rate of 9.8% over its forecast period.
The United States represents the most significant market for Deep Brain Stimulation devices globally, a position reinforced by robust demographic data and supportive regulatory frameworks. The high prevalence of neurological conditions is a primary driver. The National Institute of Neurological Disorders and Stroke (NINDS) estimates that nearly one million people in the US are living with Parkinson's disease, with approximately 60,000 new diagnoses added each year. This creates a substantial and growing patient pool for which DBS is a well-established treatment option.
Furthermore, the Centers for Disease Control and Prevention (CDC) highlights that essential tremor affects millions of Americans, many of whom are potential candidates for this therapeutic intervention when medication proves insufficient. The demographic advantage is further amplified by the aging of the US population, as detailed by the U.S. Census Bureau, which projects a considerable increase in the number of citizens over the age of 65, the demographic most at risk for Parkinson's disease.
Federal investment through the National Institutes of Health (NIH) provides critical support for the basic and clinical neuroscience research that underpins DBS innovation. This ecosystem fosters the development of next-generation devices and the exploration of new applications. The Food and Drug Administration (FDA) provides a clear, albeit rigorous, pathway for device approval, and favorable reimbursement decisions from the Centers for Medicare & Medicaid Services (CMS) are crucial for ensuring patient access.
The widespread adoption of DBS within leading academic medical centers and neurology practices across the country ensures that this advanced care is available to a significant portion of the population, solidifying the US market's leadership through a combination of demographic necessity, research infrastructure, and healthcare financing mechanisms.

The Europe Deep Brain Stimulation Devices Market
The Europe Deep Brain Stimulation Devices Market is estimated to be valued at USD 585 million in 2025 and is further anticipated to reach USD 1,170 million by 2034 at a CAGR of 8.0%.
Europe's Deep Brain Stimulation device market is a mature and sophisticated landscape, shaped by its advanced public healthcare systems and a rapidly aging demographic profile. Data from Eurostat, the statistical office of the European Union, confirms that Europe has one of the oldest populations in the world, with a steadily increasing old-age dependency ratio.
This demographic shift directly correlates with a higher incidence of age-related neurological disorders, creating a persistent and growing demand for effective treatments like DBS for conditions such as Parkinson's disease. The World Health Organization's Regional Office for Europe has consistently highlighted the rising burden of non-communicable diseases, including neurological conditions, as a key public health priority, underscoring the need for advanced therapeutic solutions.
Market dynamics across the continent are influenced by the centralized regulatory oversight of the European Medicines Agency (EMA), which, in conjunction with the new Medical Device Regulation (MDR), provides a harmonized framework for the approval and monitoring of high-risk medical devices. This ensures high standards of safety and efficacy for DBS systems available to patients.
Furthermore, many national health services and insurance providers within major economies like Germany, France, and the United Kingdom provide coverage for DBS procedures for approved indications, facilitating patient access. The presence of a dense network of world-renowned neurological research institutions, often supported by government research grants, fosters a strong environment for clinical trials and technological refinement, ensuring that European patients have access to cutting-edge neuromodulation therapies within their robust public health infrastructures.
The Japan Deep Brain Stimulation Devices Market
The Japan Deep Brain Stimulation Devices Market is projected to be valued at USD 234 million in 2025. It is further expected to witness subsequent growth in the upcoming period, holding USD 509 million in 2034 at a CAGR of 8.8%.
Japan's Deep Brain Stimulation device market is uniquely defined by its status as the world's most aged society, a demographic reality that creates a compelling and sustained need for advanced neurological care. Official statistics from the Ministry of Health, Labour and Welfare (MHLW) estimate that over 200,000 individuals in Japan are living with Parkinson's disease, a number projected to increase in lockstep with the aging population.
Data from the Statistics Bureau of Japan shows that the percentage of the population aged 65 and over is the highest globally, ensuring a large and growing patient base for movement disorder treatments. This demographic pressure acts as a powerful driver for the adoption of sophisticated medical technologies like DBS to manage the burden of chronic neurodegenerative diseases on the healthcare system.
The regulatory landscape, governed by the Pharmaceuticals and Medical Devices Agency (PMDA), is known for its rigorous review process, which can impact the speed of new device introductions. However, once approved, DBS therapies are typically covered under the national health insurance system, which provides broad access for the population.
The Japanese government, through its agencies, has identified the challenges of a super-aged society as a national priority, which includes promoting the development and deployment of advanced medical devices to maintain the quality of life for its elderly citizens. This focus, combined with the high concentration of specialized neurological centers in urban areas, supports the steady growth of the DBS market, making it a critical and established segment of Japan's advanced medical device sector.
Global Deep Brain Stimulation Devices Market: Key Takeaways
- Global Market Size Insights: The Global Deep Brain Stimulation Devices Market size is estimated to have a value of USD 3.9 billion in 2025 and is expected to reach USD 9.4 billion by the end of 2034.
- The Global Market Growth Rate: The market is growing at a CAGR of 10.4 percent over the forecasted period of 2025.
- The US Market Size Insights: The US Deep Brain Stimulation Devices Market is projected to be valued at USD 1.2 billion in 2025. It is expected to witness subsequent growth in the upcoming period as it holds USD 2.7 billion in 2034 at a CAGR of 9.8%.
- Regional Insights: North America is expected to have the largest market share in the Global Deep Brain Stimulation Devices Market with a share of about 36.2% in 2025.
- Key Players: Some of the major key players in the Global Deep Brain Stimulation Devices Market are Medtronic, Boston Scientific, Abbott, Aleva Neurotherapeutics, NeuroPace, Beijing PINS Medical, SceneRay, Renishaw, LivaNova, Newronika, and many others.
Global Deep Brain Stimulation Devices Market: Use Cases
- Parkinson's Disease: A patient with advanced Parkinson's experiences significant "off" periods where levodopa wears off. DBS stabilizes motor fluctuations, reducing tremors and dyskinesia, and restoring mobility and independence for daily activities.
- Essential Tremor: An individual with severe essential tremor finds drinking or writing socially debilitating. DBS targeted at the thalamus dramatically suppresses the tremor, allowing for restored fine motor control and improved social confidence.
- Dystonia: A patient with generalized dystonia suffers from painful, involuntary muscle contractions, causing twisting postures. DBS of the globus pallidus can alleviate these abnormal movements, reducing pain and correcting posture over time.
- Obsessive-Compulsive Disorder (OCD): For a person with severe, treatment-refractory OCD, DBS applied to specific brain circuits can interrupt the intrusive thought loops and compulsive behaviors that are unresponsive to medication and psychotherapy.
- Epilepsy: A patient with focal epilepsy continues having seizures despite multiple anti-epileptic drugs. Responsive neurostimulation, a form of closed-loop DBS, detects and delivers electrical impulses to halt seizure activity at its onset
Global Deep Brain Stimulation Devices Market: Stats & Facts
National Institute of Neurological Disorders and Stroke (NINDS)
- Nearly one million people in the United States are living with Parkinson's disease.
- Approximately 60,000 Americans are diagnosed with Parkinson's disease each year.
- Essential tremor is estimated to be eight times more common than Parkinson's disease, affecting millions of people in the US.
World Health Organization (WHO)
- Neurological disorders are the leading cause of disability and the second leading cause of death globally.
- Over 50 million people worldwide have epilepsy.
- Depression is a common mental disorder and a leading cause of disability around the world, often co-occurring with neurological conditions.
Centers for Disease Control and Prevention (CDC)
- Essential tremor is one of the most common movement disorders, affecting an estimated 7 million people in the United States of all ages and ethnicities.
- Traumatic brain injury is a major cause of death and disability in the United States, contributing to long-term neurological complications.
Parkinson's Foundation
- The combined direct and indirect cost of Parkinson's disease, including treatment, social security payments, and lost income, is estimated to be nearly $52 billion per year in the United States.
Eurostat
- More than one-fifth (21.1%) of the European Union population was aged 65 and over in 2022.
- The old-age dependency ratio in the EU is projected to increase significantly, from 33.9% in 2022 to 59.2% in 2100.
Ministry of Health, Labour and Welfare (Japan)
- It is estimated that over 200,000 people in Japan are affected by Parkinson's disease.
- Japan has the highest proportion of elderly citizens in the world, with 29.1% of its population aged 65 or over.
U.S. Census Bureau
- The population aged 65 and over in the United States is projected to grow from 58 million in 2022 to 82 million by 2050.
National Institutes of Health (NIH)
- The NIH invests over $1 billion annually in Parkinson's disease research.
- The Brain Research Through Advancing Innovative Neurotechnologies (BRAIN) Initiative, launched by the NIH, has invested hundreds of millions of dollars in research to map the brain and develop new neuromodulation therapies.
Food and Drug Administration (FDA)
- The FDA has granted Breakthrough Device designation to several next-generation DBS systems for conditions like treatment-resistant depression.
Pharmaceuticals and Medical Devices Agency (PMDA)
- The PMDA reviews and approves all medical devices for the Japanese market, including DBS systems, under its stringent safety and efficacy protocols.
Global Deep Brain Stimulation Devices Market: Market Dynamics
Driving Factors in the Global Deep Brain Stimulation Devices Market
Global Demographic Shift Towards an Aging Population
The most powerful and consistent growth driver for the DBS market is the inexorable global demographic transition towards an older population. Age stands as the single greatest risk factor for neurodegenerative diseases such as Parkinson's disease and essential tremor. As life expectancy increases worldwide, the prevalence of these chronic, progressive conditions rises in direct correlation.
This expanding patient base creates a sustained and growing demand for effective long-term treatment options that go beyond pharmacotherapy, which often loses efficacy and induces significant side effects over time. DBS is uniquely positioned as a proven surgical intervention that can manage these debilitating symptoms for many years, significantly improving quality of life and potentially reducing long-term care costs, thereby ensuring a robust and expanding market for the foreseeable future.
Substantial Public and Private Investment in Neuroscience
Substantial and sustained public and private investment in foundational neuroscience research serves as a critical catalyst for market advancement. Government-funded initiatives, such as the National Institutes of Health's BRAIN Initiative in the United States, allocate significant resources to understanding complex brain circuits and developing next-generation neurotechnology.
This foundational research directly fuels innovation in DBS, leading to the discovery of new stimulation targets and the creation of more sophisticated device architectures. Concurrently, robust clinical trial programs, often supported by device manufacturers, are essential for generating the high-quality evidence required to secure regulatory approvals for new indications, thereby continuously expanding the addressable market and validating the therapy's efficacy and safety profile to the medical community.
Restraints in the Global Deep Brain Stimulation Devices Market
High Procedural and Long-Term Cost Burden
The high overall cost of DBS therapy presents a significant barrier to widespread adoption, particularly in cost-sensitive healthcare environments and developing nations. The expense encompasses not only the high price of the implantable hardware, including leads, extensions, and the pulse generator, but also the substantial costs associated with the complex neurosurgical procedure, the requisite hospital stay, and the ongoing, long-term post-operative programming and neurological management.
Without comprehensive insurance coverage or government reimbursement, this therapy remains inaccessible to a large portion of the potential patient population. This economic burden can severely limit market penetration, making the demonstration of compelling long-term cost-effectiveness versus standard care a critical challenge for market stakeholders.
Inherent Risks of Invasive Neurosurgery
The DBS procedure carries inherent and serious risks associated with invasive brain surgery, which can deter both potential patients and their referring neurologists. These material risks include the potential for intracranial hemorrhage, stroke, infection, and inaccurate lead placement, any of which could lead to permanent neurological deficits.
Furthermore, even after a successful and uneventful implantation, patients may experience stimulation-related adverse effects such as paresthesia, muscle contractions, or mood changes. The perception of these risks, combined with the irreversibility of the surgical intervention, can slow referral rates and patient acceptance. Overcoming this restraint necessitates continuous efforts to improve surgical safety through technological advancements and robust patient education on the risk-benefit profile, which is highly favorable for carefully selected candidates.
Opportunities in the Global Deep Brain Stimulation Devices Market
Expansion into New Therapeutic Indications
A major growth frontier lies in the expansion of DBS into novel therapeutic areas beyond established movement disorders. There is active and promising clinical research exploring the efficacy of DBS for severe, treatment-resistant psychiatric conditions, including major depressive disorder and obsessive-compulsive disorder. Success in these areas would unlock a vast patient population that currently has very limited therapeutic options.
Furthermore, ongoing investigations for conditions like epilepsy, using responsive neurostimulation systems, represent a paradigm shift from continuous to on-demand brain modulation. Securing regulatory approval for these new indications would represent a significant market expansion, diversifying the application of DBS technology and bringing new hope to previously underserved patient groups.
Development of Adaptive Closed-Loop Systems
The development and commercialization of closed-loop or adaptive DBS systems present a monumental technological and commercial opportunity. Unlike current open-loop systems that deliver constant, pre-set stimulation, adaptive DBS can sense a patient's unique and fluctuating neural signals and automatically adjust stimulation parameters in real-time.
This "smart" form of neuromodulation promises to maximize therapeutic benefits while simultaneously minimizing side effects and extending the battery life of the implanted device. The successful launch of such intelligent systems would establish a premium product category, driving substantial revenue growth and establishing a new standard of care. It moves treatment from a static, one-size-fits-most approach to a truly personalized and dynamic therapy, meeting a significant unmet need for more automated and optimal chronic disease management.
Trends in the Global Deep Brain Stimulation Devices Market
Proliferation of Directional Lead Technology and Current Steering
The market is undergoing a significant transformation with the widespread adoption of directional leads and current fractionalization. Departing from traditional cylindrical leads, these advanced systems enable clinicians to precisely steer electrical current toward specific neural targets while meticulously avoiding stimulation of adjacent areas that induce side effects. This capability substantially widens the therapeutic window, delivering superior symptom control for movement disorders like Parkinson's disease while concurrently mitigating risks to speech and balance.
This technological refinement is rapidly becoming a standard expectation in new device offerings, compelling manufacturers to continuously innovate and providing neurologists with unparalleled programming flexibility to customize therapy to each patient's unique anatomy and clinical response.
Integration of Advanced Neuroimaging and Data Analytics
A dominant trend is the deep integration of sophisticated neuroimaging and data analytics into the entire DBS workflow. The utilization of high-field MRI and connectomics, the detailed study of the brain's neural connections, is revolutionizing surgical planning, shifting it from a purely anatomy-based to a connectivity-based approach. This allows surgeons to target the specific brain circuits implicated in a disease with unprecedented accuracy.
Post-operatively, the constant collection of brain signal data and stimulation parameters from the implanted device generates vast datasets. The computational analysis of this information holds the immense promise of identifying objective biomarkers for disease progression and autonomously optimizing stimulation algorithms, thereby paving the way for the next generation of adaptive, closed-loop systems that can respond in real-time to the patient's fluctuating neural state.
Global Deep Brain Stimulation Devices Market: Research Scope and Analysis
By Product Analysis
The dual-channel systems segment is projected to dominate the global Deep Brain Stimulation devices market and is expected to maintain this leadership position. This dominance is fundamentally rooted in clinical efficacy and the bilateral nature of most neurological disorders treated with DBS. Conditions like Parkinson's disease, essential tremor, and dystonia typically affect both sides of the body.
A dual-channel system, which consists of two independent implantable pulse generators (IPGs) or a single IPG with two separate channels, allows for the simultaneous and independent stimulation of both the left and right hemispheres of the brain. This bilateral approach is crucial for achieving comprehensive symptom control, such as reducing tremors in both hands or improving gait and balance, which are central to a patient's quality of life.
While single-channel systems are used for unilateral symptoms or in specific cost-containment scenarios, they represent a minority of implants because most patients present with bilateral symptoms by the time they are indicated for surgery. The clinical preference, supported by outcomes data, strongly favors the tailored, bilateral therapy that dual-channel systems provide, making them the standard of care and the revenue leader for device manufacturers. The technological sophistication and higher cost of these systems further cement their value and market share.
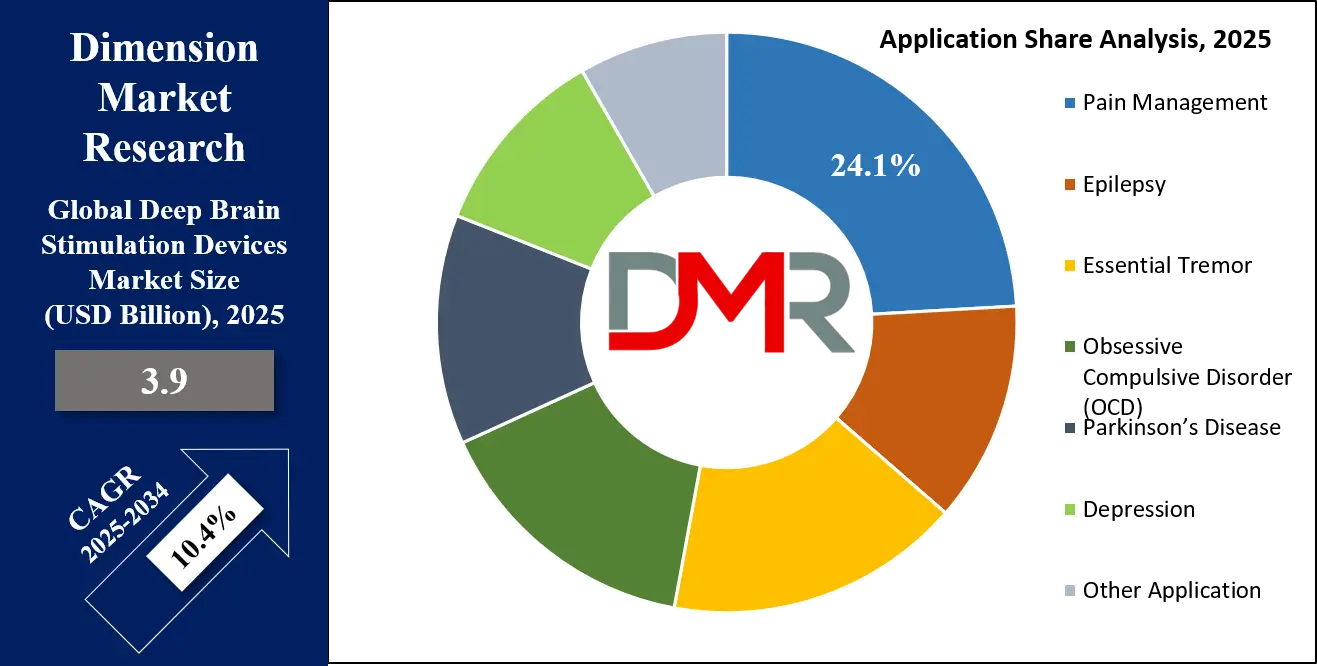
By Application Analysis
The Parkinson's disease segment is poised to be the unequivocal leader in the application category for Deep Brain Stimulation devices. This dominance is driven by a confluence of factors: a high and growing prevalence, robust clinical validation, and well-established reimbursement pathways. Parkinson's disease represents one of the most common neurological indications for which DBS is approved and performed. With a global aging population, the prevalence of this neurodegenerative disorder is increasing, creating a large and defined patient population.
Decades of extensive clinical research and long-term studies have irrefutably proven the efficacy of DBS in managing the key motor symptoms of Parkinson's, such as tremor, rigidity, and bradykinesia, particularly when patients begin to experience significant "off" periods and dyskinesias from medication. This strong evidence base has led to its inclusion in clinical practice guidelines worldwide.
Furthermore, indications like essential tremor and dystonia are also significant, but their patient populations are smaller. While emerging applications for OCD and depression represent high-growth frontiers, they are still in earlier stages of adoption. Consequently, Parkinson's disease remains the primary engine of the DBS market, accounting for the vast majority of procedures and device revenues.
By End-User Analysis
Hospitals and clinics are anticipated to constitute the dominant end-user segment for the Deep Brain Stimulation devices market. The dominance of this channel is inherent to the complex, highly specialized, and resource-intensive nature of the DBS procedure. The implantation of a DBS system is a sophisticated neurosurgical operation that requires a state-of-the-art operating room, advanced neuroimaging equipment for precise lead placement, and a multidisciplinary team comprising neurosurgeons, neurologists, and specialized nursing staff. This level of infrastructure is almost exclusively found within large tertiary care hospitals and academic medical centers.
Furthermore, the end-user relationship extends far beyond the initial surgery. The long-term management of a patient with a DBS device, including post-operative recovery, device activation, complex programming sessions to optimize stimulation parameters, managing medication adjustments, and addressing any potential complications, requires continuous oversight by trained neurologists in a clinical setting. The "home care" segment is virtually non-existent for the primary management of these devices due to the required expertise. Therefore, hospitals and clinics are not just the point of implantation; they are the central hub for the entire lifelong patient journey, solidifying their position as the critical and dominant end-user.
The Global Deep Brain Stimulation Devices Market Report is segmented on the basis of the following:
By Product
- Single-Channel Systems
- Implantable Pulse Generators
- Dual-Channel Systems
By Application
- Pain Management
- Epilepsy
- Essential Tremor
- Obsessive Compulsive Disorder (OCD)
- Parkinson’s Disease
- Depression
- Other Application
By End-User
- Hospitals & Clinics
- Homecare
- Other End User
Impact of Artificial Intelligence in the Global Deep Brain Stimulation Devices Market
- Precision Targeting and Personalized Programming: Artificial Intelligence algorithms are revolutionizing surgical planning by analyzing pre-operative MRI and CT scans to create highly detailed 3D brain maps. This allows surgeons to identify the optimal trajectory and target for electrode placement with sub-millimeter accuracy, maximizing therapeutic effect and minimizing side effects.
- Development of Adaptive Closed-Loop Systems: AI is the core enabler of the next generation of DBS: adaptive or closed-loop systems. These "smart" devices use embedded algorithms to continuously analyze a patient's local field potentials (LFPs), the brain's real-time electrical signals. The AI can detect the neural signatures of symptom onset (e.g., a tremor or seizure) and automatically adjust stimulation in response, providing therapy only when needed.
- Predictive Analytics for Patient Selection and Outcomes: Machine learning models are being trained on vast datasets of clinical, genetic, and neuroimaging data to predict which patients are most likely to respond positively to DBS therapy. By identifying predictive biomarkers, AI can assist clinicians in making more objective and data-driven patient selection decisions.
- Automated Identification of Neural Biomarkers: AI excels at finding complex patterns in large, multi-dimensional data. Researchers are using AI to sift through recorded neural signals from implanted DBS devices to discover novel biomarkers for various neurological and psychiatric diseases.
- Enhanced Surgical Robotics and Workflow Efficiency: AI is being integrated into surgical robotics used for DBS implantation. AI-guided systems can enhance surgeon precision by compensating for subtle physiological tremors and providing real-time feedback.
Global Deep Brain Stimulation Devices Market: Regional Analysis
Region with the Largest Revenue Share
North America, led by the United States, is expected to command 36.2% of the global Deep Brain Stimulation devices market share in 2025, due to a powerful convergence of technological, economic, and clinical factors. The region benefits from a high prevalence of neurological disorders, with the National Institute of Neurological Disorders and Stroke (NINDS) estimating nearly one million people in the U.S. living with Parkinson's disease alone. This creates a substantial and well-defined patient pool.
Furthermore, the region's advanced healthcare infrastructure, characterized by leading academic medical centers and specialized neurology practices, facilitates the complex DBS procedure. These institutions are often early adopters of innovative medical technologies, fostering a receptive environment for next-generation systems like those with directional leads or sensing capabilities.
Critically, robust reimbursement frameworks from both private insurers and government programs like Medicare and Medicaid provide a clear economic pathway for hospitals and patients, making the high-cost procedure accessible. This financial support, combined with substantial public and private investment in neuroscience R&D through entities like the National Institutes of Health (NIH), creates a virtuous cycle of innovation, clinical adoption, and market leadership that is difficult for other regions to match in the short term.

Region with the Highest CAGR
The Asia Pacific region is projected to exhibit the highest Compound Annual Growth Rate (CAGR) in the DBS market, driven by explosive demographic, economic, and infrastructural expansion. The primary catalyst is the rapidly aging population in key countries like Japan and China, which directly correlates with a rising incidence of age-related neurodegenerative diseases such as Parkinson's. This demographic shift is creating a vast and growing addressable patient population. Concurrently, strong economic growth across the region is translating into increased healthcare spending, greater disposable income, and the gradual expansion of public and private health insurance coverage, which is improving affordability for advanced therapies.
Governments are also actively investing in modernizing their healthcare infrastructure, leading to a proliferation of hospitals with the advanced neurosurgical capabilities required for DBS procedures. Rising awareness of neurological disorders and the availability of advanced treatment options among both physicians and patients are further stimulating demand. While market penetration is currently lower than in North America, these converging factors, a large underserved patient base, improving access, and growing clinical awareness, create a fertile ground for rapid market expansion, positioning Asia Pacific as the epicenter of future growth for the DBS industry.
By Region
North America
Europe
- Germany
- The U.K.
- France
- Italy
- Russia
- Spain
- Benelux
- Nordic
- Rest of Europe
Asia-Pacific
- China
- Japan
- South Korea
- India
- ANZ
- ASEAN
- Rest of Asia-Pacific
Latin America
- Brazil
- Mexico
- Argentina
- Colombia
- Rest of Latin America
Middle East & Africa
- Saudi Arabia
- UAE
- South Africa
- Israel
- Egypt
- Rest of MEA
Global Deep Brain Stimulation Devices Market: Competitive Landscape
The global Deep Brain Stimulation devices market is characterized by a consolidated competitive landscape, dominated by a few established medical technology giants, yet it is experiencing a dynamic shift with the entry of innovative challengers. Medtronic plc, Abbott Laboratories (acquired St. Jude Medical), and Boston Scientific Corporation are the longstanding key players, collectively holding a significant market share. Their dominance is rooted in extensive product portfolios, robust clinical evidence supporting their devices, and well-established global distribution and sales networks.
Competition among these incumbents is intense, focusing on technological differentiation through features like directional steering, rechargeable batteries, and advanced magnetic resonance imaging (MRI) compatibility. However, the landscape is evolving. A new wave of specialized companies, such as Aleva Neurotherapeutics, which is pioneering direct targeting technology, and Functional Neuromodulation Ltd., are entering the fray. These entrants often focus on niche applications or disruptive technologies, particularly in the realm of personalized and adaptive stimulation.
The competitive dynamics are further shaped by strategic activities beyond pure product sales. Key players are actively engaging in mergers and acquisitions to acquire novel technologies, forming research collaborations with leading academic institutions to explore new indications, and investing heavily in large-scale clinical trials to expand their approved labeling and secure a competitive edge in the rapidly advancing field of neuromodulation.
Some of the prominent players in the Global Deep Brain Stimulation Devices Market are:
- Medtronic
- Boston Scientific
- Abbott (St. Jude Medical)
- Aleva Neurotherapeutics
- NeuroPace
- Beijing PINS Medical
- SceneRay
- Renishaw
- LivaNova
- Newronika
- Synaptic Medical (Shenzhen)
- Alpha Omega Engineering
- AlphaDBS Solutions (Deep Brain Innovations)
- NeuroSigma
- Nexstim
- BrainsWay
- Adaptive Neuromodulation
- Soterix Medical
- CorTec
- Synchron.
- Other Key Players
Recent Developments in the Global Deep Brain Stimulation Devices Market
- May 2024: Boston Scientific's ACCELERATE trial shows positive one-year results for its novel Parkinson's disease DBS system, demonstrating significant symptom improvement and a strong safety profile in treated patients.
- March 2024: The American Academy of Neurology's annual meeting featured new research on advanced DBS programming algorithms and long-term patient outcomes, highlighting trends toward personalized therapy.
- February 2024: Medtronic commenced a pivotal clinical trial investigating the efficacy of its Deep Brain Stimulation technology for patients with severe, treatment-resistant major depressive disorder.
- January 2024: Abbott presented real-world evidence confirming the sustained long-term effectiveness of its directional DBS systems in controlling essential tremor symptoms and improving patients' quality of life.
- November 2023: At the Society for Neuroscience meeting, dedicated symposia explored advancements in closed-loop DBS systems and the identification of neural biomarkers for adaptive neuromodulation therapies.
- October 2023: Boston Scientific secured Japanese regulatory approval for its Vercise DBS platform, expanding access to advanced Parkinson's disease treatment within the key Asia-Pacific market.
- September 2023: The International Neuromodulation Society's European meeting focused on technical innovations in DBS lead design, including new materials and configurations for precise current steering.
- August 2023: Aleva Neurotherapeutics successfully secured a significant new venture funding round to accelerate the commercial rollout of its direct targeting DBS system for movement disorders.
- July 2023: Abbott launched its "Reclaim Your Moment" patient awareness campaign across Europe to educate individuals with movement disorders about modern DBS treatment options.
- June 2023: Medtronic announced a strategic collaboration with a leading European university to co-develop artificial intelligence-powered programming tools for optimizing DBS therapy settings.
Report Details
| Report Characteristics |
| Market Size (2025) |
USD 3.9 Bn |
| Forecast Value (2034) |
USD 9.4 Bn |
| CAGR (2025–2034) |
10.4% |
| The US Market Size (2025) |
USD 1.2 Bn |
| Historical Data |
2019 – 2024 |
| Forecast Data |
2026 – 2034 |
| Base Year |
2024 |
| Estimate Year |
2025 |
| Report Coverage |
Market Revenue Estimation, Market Dynamics, Competitive Landscape, Growth Factors, etc. |
| Segments Covered |
By Product (Single-Channel Systems, Implantable Pulse Generators, Dual-Channel Systems), By Application (Pain Management, Epilepsy, Essential Tremor, Obsessive Compulsive Disorder (OCD), Parkinson’s Disease, Depression, Other Application), By End-User (Hospitals & Clinics, Homecare, Other End User)
|
| Regional Coverage |
North America – US, Canada; Europe – Germany, UK, France, Russia, Spain, Italy, Benelux, Nordic, Rest of Europe; Asia-Pacific – China, Japan, South Korea, India, ANZ, ASEAN, Rest of APAC; Latin America – Brazil, Mexico, Argentina, Colombia, Rest of Latin America; Middle East & Africa – Saudi Arabia, UAE, South Africa, Turkey, Egypt, Israel, Rest of MEA |
| Prominent Players |
Medtronic, Boston Scientific, Abbott, Aleva Neurotherapeutics, NeuroPace, Beijing PINS Medical, SceneRay, Renishaw, LivaNova, Newronika, Synaptic Medical, Alpha Omega Engineering, AlphaDBS Solutions, NeuroSigma, Nexstim, BrainsWay, Adaptive Neuromodulation, Soterix Medical, CorTec, Synchron, and Other Key Players |
| Purchase Options |
We have three licenses to opt for: Single User License (Limited to 1 user), Multi-User License (Up to 5 Users), and Corporate Use License (Unlimited User) along with free report customization equivalent to 0 analyst working days, 3 analysts working days, and 5 analysts working days respectively. |
Frequently Asked Questions
The Global Deep Brain Stimulation Devices Market size is estimated to have a value of USD 3.9 billion in 2025 and is expected to reach USD 9.4 billion by the end of 2034.
The market is growing at a CAGR of 10.4 percent over the forecasted period of 2025.
The US Deep Brain Stimulation Devices Market is projected to be valued at USD 1.2 billion in 2025. It is expected to witness subsequent growth in the upcoming period as it holds USD 2.7 billion in 2034 at a CAGR of 9.8%.
North America is expected to have the largest market share in the Global Deep Brain Stimulation Devices Market with a share of about 36.2% in 2025.
Some of the major key players in the Global Deep Brain Stimulation Devices Market are Medtronic, Boston Scientific, Abbott, Aleva Neurotherapeutics, NeuroPace, Beijing PINS Medical, SceneRay, Renishaw, LivaNova, Newronika, and many others.