Market Overview
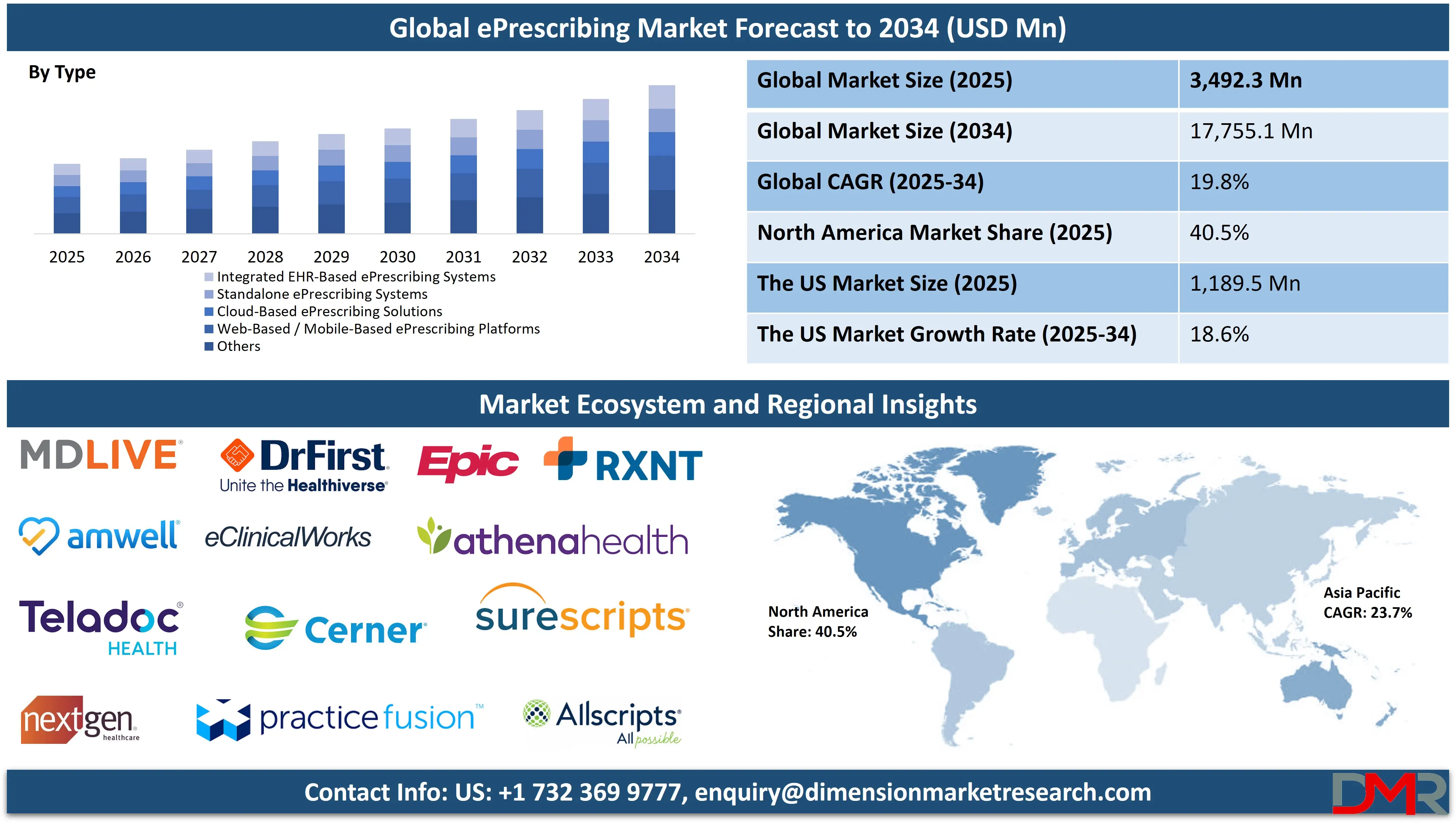
The Global ePrescribing Market is poised for a period of exceptional growth, projected to reach USD 3,492.3 million in 2025 and accelerate at a formidable CAGR of 19.8% from 2025 to 2034, ultimately attaining a monumental value of USD 17,755.1 million by 2034.
This explosive expansion is fundamentally driven by a powerful global convergence of factors, including concerted government mandates pushing for the digitization of health records, an unwavering focus on enhancing patient safety by eliminating medication errors, and the overarching need to streamline administrative workflows within increasingly burdened healthcare systems. ePrescribing serves as a critical technological linchpin in this transformation, enabling healthcare providers to electronically generate, sign, and transmit prescriptions directly to pharmacies in a secure, seamless, and efficient manner.
This digital process drastically reduces the risks associated with illegible handwriting, manual data entry mistakes, and paper-based communication delays, while simultaneously integrating vital clinical decision support tools that check for drug-drug interactions, allergy conflicts, and formulary compliance at the point of care.
The model effectively addresses some of the most persistent and costly challenges in modern healthcare, including medication non-adherence, which is a leading cause of poor chronic disease outcomes; prescription fraud, particularly concerning controlled substances; and profound administrative inefficiencies that consume valuable clinical time. The market is being further propelled by a wave of technological advancements that are enriching the capabilities of ePrescribing systems.
These include sophisticated AI-driven clinical decision support that offers personalized therapy recommendations; highly interoperable cloud platforms that facilitate data exchange across different healthcare settings; intuitive mobile ePrescribing apps that provide flexibility for providers; blockchain technology for ensuring unparalleled prescription security and integrity; and IoT-enabled medication adherence tools that connect prescribing to patient behavior monitoring.
While the growth trajectory is strong, the market does face notable headwinds, including the high initial implementation and ongoing maintenance costs that can be prohibitive for smaller practices, persistent interoperability challenges between disparate health information systems, valid data privacy and security concerns, and a degree of clinical resistance to changing established workflows.
Nevertheless, the powerful confluence of supportive regulatory policies, relentless technological innovation, and the undeniable clinical and operational benefits positions ePrescribing not merely as a convenient tool, but as a central and indispensable driver of global healthcare digitization through 2034 and beyond.
The US ePrescribing Market
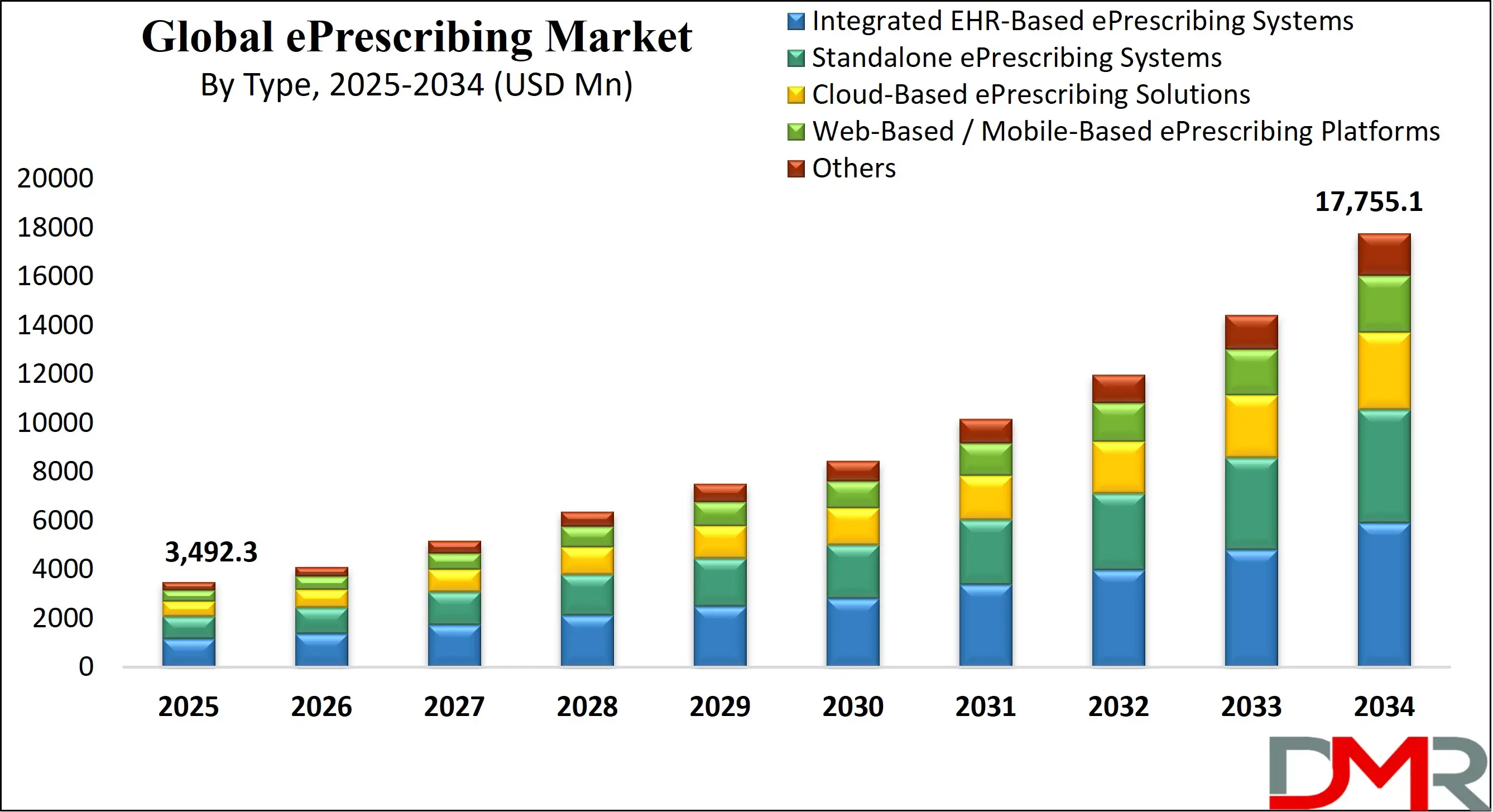
The U.S. ePrescribing Market stands as the global frontrunner, projected to reach USD 1,189.5 million in 2025 and grow at a robust CAGR of 18.6%, reaching a substantial USD 5,509.3 million by 2034. The United States' leadership position is underpinned by a deeply entrenched digital health ecosystem, characterized by near-ubiquitous Electronic Health Record (EHR) adoption among office-based physicians, which exceeds 85%.
This foundation is fortified by a robust and supportive regulatory framework, most notably the Drug Enforcement Administration's (DEA) mandate for Electronic Prescribing for Controlled Substances (EPCS), which has been a critical catalyst for widespread adoption.
Furthermore, influential programs from the Centers for Medicare & Medicaid Services (CMS), such as the Promoting Interoperability program (formerly Meaningful Use), have created powerful financial incentives and penalties that have systematically driven integration into standard clinical practice.
Major, influential health systems like Kaiser Permanente, the Mayo Clinic, and the Cleveland Clinic have not only adopted but have fully optimized ePrescribing, seamlessly weaving it into their comprehensive EHR and patient engagement strategies to enhance medication management across the entire care continuum.
The U.S. reimbursement environment is exceptionally favorable, with Medicare Part D plans requiring ePrescribing, and both Medicaid and private insurers providing coverage for these digital services, making the business case for adoption financially compelling for providers and health systems alike.
The legislative landscape has been further solidified by the SUPPORT Act, which mandates EPCS for all controlled substance prescriptions under Medicare, a move widely credited with significantly reducing opportunities for prescription forgery and diversion. Concurrently, national initiatives led by the Office of the National Coordinator for Health IT (ONC) actively promote advanced interoperability and secure, standardized data exchange, thereby strengthening the underlying infrastructure that makes nationwide ePrescribing possible.
The market is currently being reshaped by the rapid ascent of cloud-based ePrescribing platforms that offer greater scalability and lower upfront costs, the proliferation of mobile provider applications that enable prescribing from any location, deep pharmacy integration tools that streamline the fulfillment process, and the emergence of AI-powered medication adherence systems that use predictive analytics to identify and support patients at risk of non-compliance, collectively reinforcing the country's status as a global beacon in digital medication management.
The Europe ePrescribing Market
The Europe ePrescribing Market represents a sophisticated and rapidly maturing landscape, projected to be valued at approximately USD 720 million in 2025 and expected to surge to around USD 3,650 million by 2034, growing at a CAGR of about 19.2% over the forecast period.
Europe's advanced position is anchored by a combination of strong, forward-thinking regulatory frameworks at both the national and European Union level, ambitious cross-border health initiatives, and comprehensive national digital health strategies that explicitly promote the transition to paperless prescribing.
Pioneering countries such as the U.K., Germany, France, Estonia, Finland, and the Netherlands have achieved widespread adoption, driven largely by their national health services and ambitious EU-level interoperability projects like the eHealth Digital Service Infrastructure (eHDSI), which is gradually enabling secure ePrescription exchange for citizens traveling across member states.
The U.K.'s NHS Digital, for instance, has been instrumental in implementing ePrescribing across both primary and secondary care settings, leading to measurable reductions in transcription errors, improved coordination between general practitioners and community pharmacies, and more efficient medication reconciliation processes.
The region's demographic shift towards an aging population, which corresponds with a high and growing burden of chronic diseases requiring complex, long-term medication regimens, places a premium on medication safety and efficiency, further propelling ePrescribing uptake.
This is complemented by a deep-seated cultural and regulatory emphasis on patient safety and data privacy, embodied by regulations like the GDPR. Significant funding mechanisms, including the EU4Health Program and Horizon Europe, alongside substantial national digital transformation funds, are actively channeling resources into the adoption of interoperable ePrescribing systems, the expansion of cross-border prescription exchange capabilities, and the development of secure patient access portals that allow individuals to view their medication histories.
Hospitals, clinics, and community pharmacies across the continent are increasingly deploying advanced cloud-based prescribing systems, integrated clinical decision support modules, and real-time prescription monitoring tools to track controlled substances. With its strong data governance models, high degree of digital maturity in healthcare, and sustained investment in creating a region-wide health data exchange ecosystem, Europe is firmly established as one of the most advanced and integrated regions for ePrescribing penetration globally.
The Japan ePrescribing Market
The Japan ePrescribing Market is on a trajectory of remarkably rapid growth, anticipated to be valued at approximately USD 98 million in 2025 and expected to attain nearly USD 580 million by 2034, expanding at an impressive CAGR of about 21.5% during the forecast period.
Japan's market dynamics are uniquely shaped by its unprecedented population aging, with nearly 30% of its citizens aged 65 or above, which creates an immense and sustained demand for sophisticated chronic medication management solutions, support for managing polypharmacy (the use of multiple medications), and technologies that enhance overall prescription safety for a vulnerable demographic.
The Ministry of Health, Labour and Welfare (MHLW) is a proactive force in this domain, actively supporting ePrescribing adoption through well-defined national digital health strategies. These initiatives are designed to enable secure electronic prescription transmission, incorporate AI-driven drug interaction checks to prevent adverse events, and provide cloud-based access to comprehensive medication histories for authorized providers.
Japan's renowned leadership in fields such as healthcare robotics, the Internet of Things (IoT), and precision medicine serves as a powerful accelerator for innovation, leading to the development of highly advanced, integrated ePrescribing-EHR systems that are both intelligent and user-friendly.
The country's overarching vision for "Smart Healthcare", championed by technology giants like Fujitsu, NEC, and the healthcare platform M3, strategically integrates ePrescribing with a broader ecosystem that includes telemedicine platforms, extensive pharmacy networks, and emerging home care services.
In highly urbanized regions such as Tokyo and Osaka, there is a focused deployment of AI-assisted prescribing tools within clinics and hospital outpatient departments, while rural prefectures are leveraging cloud-based ePrescribing systems to connect remote providers with centralized pharmacy hubs, thus overcoming geographic barriers to care.
Japan's deeply ingrained cultural emphasis on precision, efficiency, and technological adoption, combined with its world-class capabilities in health information technology, positions the country not just as an adopter, but as a high-growth innovator and a critical market to watch in the global ePrescribing landscape.
Global ePrescribing Market: Key Takeaways
- Strong Global Market Growth Outlook: The Global ePrescribing Market is on a steep growth curve, expected to be valued at USD 3,492.3 million in 2025 and projected to reach a staggering USD 17,755.1 million by 2034. This rapid expansion is fundamentally supported by the escalating demand for digital solutions that can streamline the prescription process, reduce costly medication errors.
- High CAGR Driven by Digital Health Adoption: The market is poised to grow at an impressive Compound Annual Growth Rate (CAGR) of 20.1% from 2025 to 2034. This growth is fueled by a powerful combination of factors, including accelerated EHR integration across care settings, forceful government mandates and incentives.
- Strong Growth Trajectory in the United States: The U.S. ePrescribing Market, a projected to be dominant force in the global arena, stands at USD 1,189.5 million in 2025 and is projected to reach USD 5,509.3million by 2034, expanding at a CAGR of 18.6%.
- North America Maintains Regional Dominance: North America is expected to command a commanding 40.5% of the global market share in 2025. This leadership is supported by an unrivalled ecosystem that includes advanced healthcare IT infrastructure, the highest rates of EHR adoption in the world.
- Rapid Advancement in ePrescribing Technologies: The market is being continuously transformed by a wave of technological innovations. These include sophisticated AI-based drug interaction and allergy alerts that learn from data, cloud-based prescription networks that connect entire regions, mobile ePrescribing apps that offer unprecedented flexibility to clinicians, blockchain implementations that provide immutable security for controlled substance prescriptions.
- Growing Burden of Medication Errors Boosts Adoption: A rising global focus on patient safety, driven by alarming statistics on medication-related harm, is a primary catalyst for adoption. Simultaneously, the healthcare industry's intensified efforts to improve medication adherence to achieve better chronic disease outcomes.
Global ePrescribing Market: Use Cases
- Primary Care ePrescribing: In the backbone of the healthcare system, primary care physicians routinely generate and transmit prescriptions electronically during patient visits. This process is deeply integrated with the patient's EHR, allowing for instant access to their full medical history, automated drug-allergy checks, real-time formulary support to ensure insurance coverage, and the ability to select the patient's preferred pharmacy with a single click, dramatically streamlining the entire workflow.
- Specialty Medication Management: Specialists in fields such as oncology, psychiatry, and rheumatology rely heavily on ePrescribing for managing complex and often high-cost medication regimens. These systems are equipped with built-in support for handling the cumbersome prior authorization process, providing detailed clinical documentation requirements, and facilitating direct coordination with specialty pharmacies to ensure patients receive their vital therapies without dangerous delays.
- Telemedicine Prescriptions: The explosion of telehealth has made integrated ePrescribing an absolute necessity. Telehealth platforms now seamlessly incorporate ePrescribing functionalities, enabling remote providers to conduct virtual consultations and then send prescriptions directly and securely to a pharmacy of the patient's choice, creating a continuous and convenient care experience that mirrors an in-person visit.
- Hospital Discharge Workflow: Hospitals utilize ePrescribing as a critical tool to streamline and error-proof the discharge process. By generating discharge prescriptions electronically, they eliminate dangerous transcription errors that can occur when moving from inpatient to outpatient care, ensure accurate medication reconciliation, and guarantee continuity of care by directly notifying the community pharmacy before the patient even leaves the hospital grounds.
- Controlled Substance ePrescribing (EPCS): This specialized application addresses a critical public health issue. Providers use EPCS systems, which incorporate rigorous identity proofing and two-factor authentication, to electronically prescribe scheduled drugs in full compliance with DEA (in the U.S.) and other national regulations. This digital process creates an immutable audit trail, drastically reduces forgery and diversion, and adds a powerful layer of safety to the prescribing of opioids and other controlled medications.
Global ePrescribing Market: Stats & Facts
- The World Health Organization (WHO) has highlighted that medication-related errors inflict an estimated annual cost of USD 42 billion worldwide, a staggering figure that underscores the immense economic and human burden of preventable mistakes. ePrescribing systems directly combat this by incorporating automated dose checking, robust allergy alerts, and comprehensive drug interaction warnings, which is particularly crucial for the growing population of polypharmacy patients, among whom over 30% experience adverse drug events.
- The U.S. Centers for Disease Control and Prevention (CDC) has reported that the implementation of ePrescribing can reduce medication errors by up to 50%. This is achieved by systems proactively flagging potential allergies, therapeutic duplications, and incorrect dosages before the prescription is ever transmitted to the pharmacy, thereby substantially improving patient safety in environments where over 70% of all patient visits result in a prescription being written.
- The U.S. Drug Enforcement Administration (DEA) has instituted a mandate for Electronic Prescribing for Controlled Substances (EPCS) for all prescriptions covered under Medicare Part D, a policy fully effective from 2025. This regulation is a massive driver of adoption in a market where over 15% of all prescriptions are for controlled substances, compelling providers to invest in and utilize certified EPCS technology.
- The European Commission has noted that cross-border ePrescribing activity within the European Union has grown by an remarkable over 200% since 2022. This growth is facilitated by sophisticated systems like the eHealth Digital Service Infrastructure (eHDSI), which enables secure and seamless prescription exchange for travelers and expatriates across over 20 participating member states, promoting continuity of care throughout the EU.
- According to data from the Health IT Dashboard, over 90% of U.S. pharmacies are now fully enabled to receive electronic prescriptions. This near-universal connectivity enables a seamless data flow between prescribing providers and dispensing pharmacists, which has been shown to reduce time-consuming phone calls and error-prone faxes by up to 70%, freeing up both clinical and administrative staff for more value-added tasks.
- The National Institute for Health and Care Excellence (NICE) in the U.K. has published evidence indicating that ePrescribing contributes to improved medication adherence by up to 20%. This improvement is facilitated by features such as timely electronic refill requests, automated patient reminder systems, and direct patient access to their digital medication records, which together empower individuals to better manage their own health.
Global ePrescribing Market: Market Dynamic
Driving Factors in the Global ePrescribing Market
Government Regulations and Incentives
Stringent government mandates and strategic financial incentives represent the most powerful drivers accelerating global ePrescribing adoption. Landmark programs such as the U.S. Promoting Interoperability program (the evolution of Meaningful Use), the DEA's definitive EPCS rule, the European eHealth Network's directives, and ambitious national digital health strategies across the Asia-Pacific region collectively compel healthcare providers to adopt certified ePrescribing technology.
The primary objectives of these regulations are multifaceted: to drastically reduce prescription fraud and forgery, especially for controlled substances; to create transparent and auditable digital trails for all prescribed medications; and to fundamentally enhance patient safety by embedding clinical checks into the prescribing process.
Rising Focus on Patient Safety and Medication Error Reduction
The urgent and global focus on mitigating medication errors, which are a significant cause of patient morbidity, mortality, and avoidable healthcare costs, is a fundamental driver for ePrescribing.
These digital systems are specifically engineered to minimize errors through a multi-layered safety approach that includes automated, real-time checks for dangerous drug-drug interactions, proactive allergy alerts that cross-reference a patient's known allergies with a new prescription, dose range validation based on age, weight, and clinical condition, and the generation of legible, standardized, and unambiguous prescriptions.
The integration of these systems with advanced clinical decision support (CDS) tools provides evidence-based therapy recommendations and alternative options, further reducing the likelihood of adverse drug events.
Restraints in the Global ePrescribing Market
High Implementation and Maintenance Costs
The significant financial investment required for ePrescribing systems poses a substantial barrier to adoption, particularly for smaller medical practices, independent clinics, and healthcare facilities in low- and middle-income countries.
The initial costs are multifaceted, encompassing not only the licensing fees for the software itself but also the necessary hardware upgrades, comprehensive training programs for clinical and administrative staff, and the complex, often costly, process of integrating the new system with existing EHRs and practice management software. Beyond these upfront capital expenditures, ongoing expenses for software updates, technical support, cybersecurity measures, and maintaining compliance with evolving regulatory standards create a continuous financial burden.
Interoperability and Data Standardization Challenges
A critical and persistent challenge hindering the seamless effectiveness of ePrescribing is the lack of universal data standards and robust interoperability between the multitude of different EHR systems, pharmacy management software, and health information networks.
Variations in formulary requirements across hundreds of different insurance plans, discrepancies in medication databases used by different systems, and the use of differing clinical terminology (e.g., SNOMED CT vs. RxNorm) across regions and software platforms can lead to prescription transmission failures, misinterpretation of instructions, or incomplete data transfer.
Without robust, widely adopted health information exchange (HIE) networks that can reliably translate and route this data, the full potential of ePrescribing such as comprehensive medication history access and seamless cross-continuum care coordination cannot be fully realized. This
Opportunities in the Global ePrescribing Market
Expansion in Emerging Economies
Emerging markets present arguably the most substantial long-term growth opportunities for the ePrescribing market. This potential is driven by a powerful convergence of factors: explosive smartphone penetration that is leapfrogging traditional IT infrastructure, ambitious government-led digital health missions (such as India's National Digital Health Mission and Brazil's Conecte SUS), and a rapidly rising burden of chronic diseases that require long-term medication management.
Countries across Asia-Pacific, Latin America, and Africa are increasingly launching national ePrescribing initiatives as integral components of their broader health digitization and universal health coverage agendas. The development and promotion of affordable cloud-based Software-as-a-Service (SaaS) solutions and mobile-first ePrescribing apps are particularly well-suited for rapid scaling in resource-constrained settings, as they minimize upfront costs and hardware requirements.
AI and Advanced Analytics Integration
The integration of Artificial Intelligence (AI) and machine learning (ML) algorithms into ePrescribing systems is unlocking a new frontier of opportunities that extend far beyond basic electronic transmission.
These advanced capabilities enable predictive analytics that can forecast patient-specific adherence risks based on historical behavior and social determinants of health, support personalized medicine by suggesting therapies tailored to an individual's genetic profile or comorbidities, and empower population health management by analyzing aggregate prescribing data to identify regional trends, outlier prescribing patterns, and opportunities for quality improvement interventions. AI can also analyze vast datasets to identify cost-saving opportunities by suggesting therapeutically equivalent but more cost-effective alternative medications.
Trends in the Global ePrescribing Market
Cloud-Based ePrescribing Solutions
Cloud-based ePrescribing platforms are experiencing a massive surge in traction and are becoming the deployment model of choice for many organizations. Their appeal lies in inherent advantages such as superior scalability, which allows health systems to easily add new users or locations; significantly lower upfront capital expenditure, as they operate on a subscription model; ease of updates and maintenance, with vendors managing all technical upgrades remotely; and remote accessibility, enabling authorized providers to securely access the system from any internet-connected device.
These cloud-native systems are engineered to support real-time prescription transmission, seamless bidirectional integration with pharmacy networks, and the maintenance of a centralized, always-up-to-date medication history for each patient.
Mobile ePrescribing Applications
The proliferation of mobile health (mHealth) is profoundly influencing ePrescribing, with a growing trend towards powerful, full-featured mobile applications that allow providers to generate, digitally sign, and send prescriptions directly from their smartphones or tablets.
These apps are increasingly sophisticated, often incorporating features like barcode scanning to quickly input medication data, voice-to-text entry for hands-free operation, offline functionality that syncs data once a connection is restored, and secure two-factor authentication specifically designed to meet the stringent security requirements for EPCS. Mobile ePrescribing significantly enhances workflow flexibility for physicians, allowing them to manage prescriptions outside of the traditional office setting for example, while on hospital rounds or when responding to patient messages after hours.
Global ePrescribing Market: Research Scope and Analysis
By Type Analysis
Integrated EHR Systems are projected to continue their dominance over the global ePrescribing market throughout the forecast period. This supremacy is due to their unparalleled ability to provide seamless workflow integration, granting clinicians comprehensive, one-click access to the patient's entire health record including problem lists, lab results, and previous notes directly at the point of prescribing. This holistic view enables safer, more informed, and highly efficient prescribing decisions.
Integrated systems are the preferred solution for hospitals, large multi-specialty clinics, and accountable care organizations because they natively support the attainment of meaningful use criteria, facilitate sophisticated electronic quality reporting, and are essential for enabling coordinated care across different providers and settings. The dominance of this segment is powerfully reinforced by government incentives that explicitly favor the adoption of certified, interoperable EHR-ePrescribing systems and the broader industry trend towards consolidated, unified digital health platforms that avoid the silos and inefficiencies of managing multiple standalone applications.
Standalone Systems continue to hold a significant, though gradually contracting, share of the market. They remain relevant in specific niches, such as smaller medical practices that have not yet adopted a full EHR, or in specialized settings like certain retail health clinics or pharmacy-based prescribing scenarios where a full-featured EHR is not required or is cost-prohibitive. These systems focus exclusively on the functions of prescription generation, transmission, and pharmacy communication, often making them more affordable and simpler to implement than their integrated counterparts. However, as the healthcare industry moves inexorably toward integrated, value-based care models that demand a comprehensive view of the patient, the market share for standalone ePrescribing systems is expected to gradually decline in favor of more holistic solutions.
By Application Analysis
Medication Management is poised to stands as the largest and most dominant application segment within the global ePrescribing market. This broad category encompasses the entire medication lifecycle, including new prescription generation, refill request and authorization management, complex medication reconciliation processes (especially during care transitions), and ensuring formulary compliance to optimize drug coverage and patient cost. ePrescribing systems are fundamentally designed to streamline these interconnected processes, thereby reducing the administrative burden on clinicians and staff, minimizing opportunities for error at every step, and profoundly enhancing patient safety.
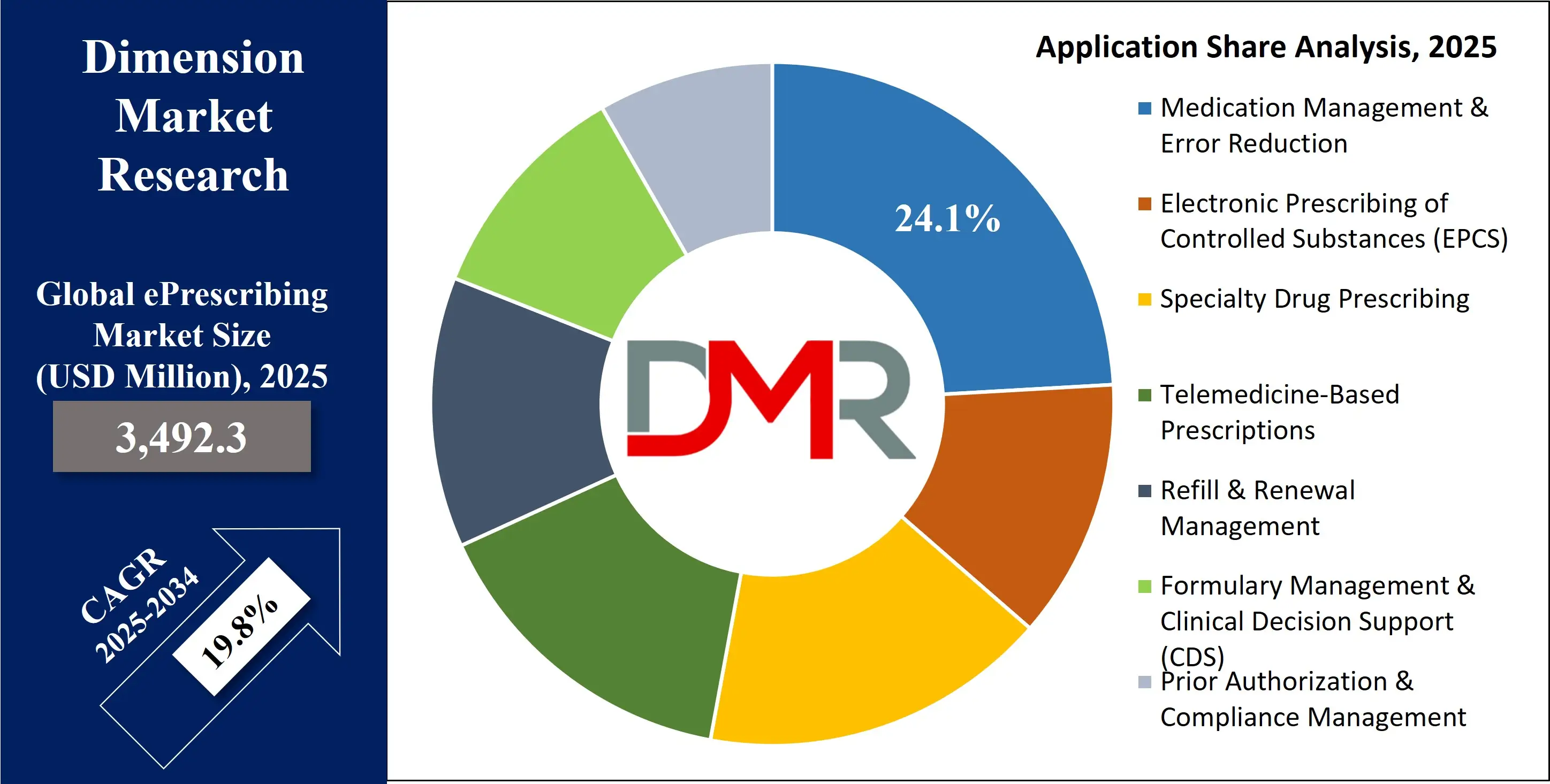
The critical role of effective medication management in the context of chronic disease care, routine primary care, and complex hospital settings ensures this segment's continued dominance. Its leading position is further solidified by government and payer programs that explicitly focus on improving medication adherence as a key quality metric and on implementing technologies proven to reduce medication errors, making it a perpetual focal point for investment and innovation.
Controlled Substance Prescribing (EPCS) is firmly established as the second-largest application segment, and its growth is directly propelled by stringent and evolving regulatory requirements aimed at combating the ongoing public health crisis of prescription drug abuse. EPCS systems are not standard ePrescribing tools; they are specially engineered with enhanced security protocols, including rigorous identity proofing for prescribers, mandatory two-factor authentication for each transaction, and the creation of detailed, immutable audit trails for compliance monitoring.
The U.S. DEA's mandate for EPCS, along with the implementation of similar stringent regulations in countries like Canada and across Europe, serves as the primary growth engine for this segment. As regulators worldwide continue to tighten controls on opioids and other scheduled medications, the adoption of certified EPCS technology becomes not just a best practice, but a legal necessity for a vast number of providers.
By End User Analysis
Hospitals and Health Systems are anticipated to remain the dominant end-user segment in the ePrescribing market. This is a direct result of their immense prescription volumes, highly complex medication workflows that span inpatient, outpatient, and emergency departments, and their role as early adopters of integrated health information technology systems.
Hospitals leverage ePrescribing for a wide range of functions, including inpatient medication orders that replace error-prone verbal and handwritten orders, discharge prescriptions that ensure a safe transition to home, and outpatient clinic prescriptions that require integration with the main hospital EHR. The imperative for achieving the highest levels of medication safety, ensuring strict regulatory compliance with standards from bodies like The Joint Commission, and driving operational efficiency in a high-stakes environment all converge to strengthen the hospital segment's dominant market position.
Office-Based Physicians constitute the second-largest end-user segment, a status driven by the sheer volume of prescriptions generated in the ambulatory care setting, which represents the majority of all prescriptions written. ePrescribing integrates seamlessly into the workflow of primary care and specialty practices, enabling providers to send prescriptions directly to the pharmacy during or immediately after the patient visit, thereby enhancing convenience and closing care gaps. The growth in this segment is vigorously supported by the continuous evolution of EHR vendors who bundle ePrescribing as a core feature, ongoing government incentives that encourage use in ambulatory settings, and the relentless expansion of pharmacy networks that are capable of receiving electronic prescriptions, ensuring that virtually every prescription can be sent digitally.
The Global ePrescribing Market Report is segmented on the basis of the following:
By Type
- Integrated EHR-Based ePrescribing Systems
- Standalone ePrescribing Systems
- Cloud-Based ePrescribing Solutions
- Web-Based / Mobile-Based ePrescribing Platforms
- Others
By Application
- Medication Management & Error Reduction
- Electronic Prescribing of Controlled Substances (EPCS)
- Specialty Drug Prescribing
- Telemedicine-Based Prescriptions
- Refill & Renewal Management
- Formulary Management & Clinical Decision Support (CDS)
- Prior Authorization & Compliance Management
By End User
- Hospitals & Health Systems
- Office-Based Physicians
- Pharmacies
- Telemedicine Platforms
- Ambulatory Care Centers
Impact of Artificial Intelligence in the Global ePrescribing Market
- AI for Drug Interaction and Allergy Alerts: AI significantly enhances clinical decision support by moving beyond simple rule-based checks. It can analyze complex, patient-specific data including genetics, comorbidities, and concurrent medications to flag subtle but potentially dangerous drug-drug interactions, identify non-obvious allergy conflicts, and warn of duplicate therapies in real-time, thereby providing a sophisticated safety net that reduces adverse drug events.
- AI-Driven Prescription Pattern Analysis: Machine learning algorithms can analyze vast datasets of aggregated, de-identified prescribing data to identify outlier patterns that may indicate errors, unnecessary variations in care, or opportunities for optimization. This can help health systems enforce formulary adherence more effectively, suggest therapeutically equivalent but more cost-effective alternative medications, and support antimicrobial stewardship programs.
- Predictive Adherence Support: By analyzing historical prescription fill data, social determinants of health, and therapy complexity, AI can generate predictive risk scores for individual patients who are likely to become non-adherent. This enables care teams to initiate proactive interventions, such as automated reminders, pharmacist consultations, or social work support, before a patient misses a dose, thereby improving long-term health outcomes.
- Automated Prior Authorization: AI is revolutionizing the notoriously cumbersome prior authorization process. Intelligent systems can auto-populate complex forms, instantly check a patient's insurance plan requirements against the prescribed medication, and even electronically submit prior authorization requests directly to payers, dramatically reducing administrative delays and ensuring patients receive timely access to necessary treatments.
- Voice-Activated Prescribing: Leveraging advanced natural language processing (NLP), AI-powered voice recognition systems allow physicians to dictate prescriptions hands-free. This technology integrates with clinical workflows to convert speech directly into structured prescription orders within the EHR, improving workflow efficiency, reducing data entry errors, and allowing for greater eye contact and patient engagement during consultations.
Global ePrescribing Market: Regional Analysis
Region with the Largest Revenue Share
North America is projected to dominate the Global ePrescribing Market, holding a commanding 40.5% of the market share by the end of 2025. This leadership is attributable to an unrivalled ecosystem that features the world's most advanced healthcare IT infrastructure, exceptionally high penetration rates of Electronic Health Records (EHRs) in both hospital and ambulatory settings, and a strong, clear regulatory framework that actively encourages adoption. The United States is the primary engine of this dominance, driven by pivotal regulations such as the DEA's EPCS mandates and Medicare Part D ePrescribing requirements, which have created a compliance-driven market. Furthermore, the U.S. benefits from a deeply integrated pharmacy network that is virtually universally capable of receiving electronic prescriptions.
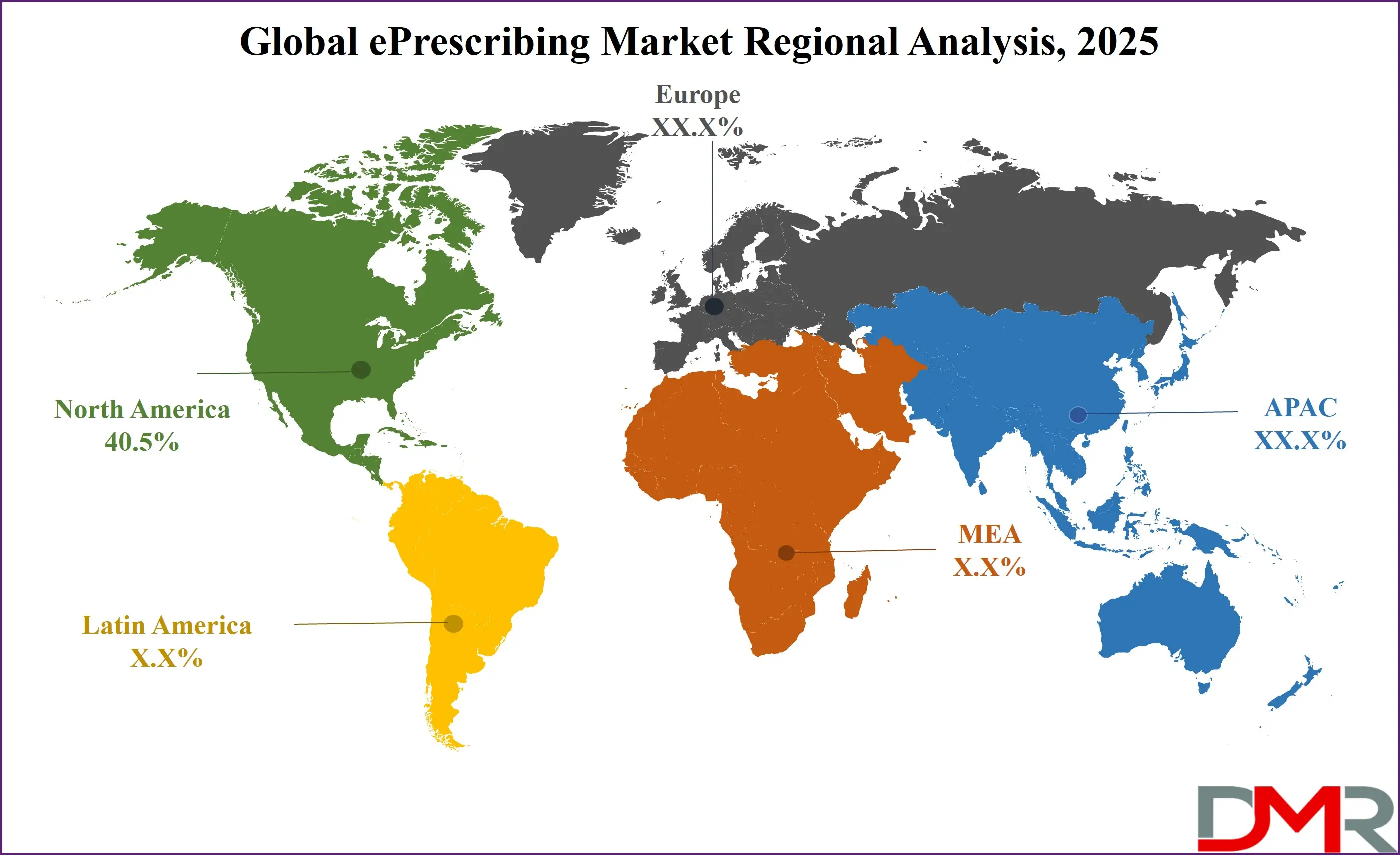
Major EHR vendors like Epic, Cerner, and Allscripts have made ePrescribing a foundational, seamlessly integrated module within their platforms, which has been instrumental in driving widespread adoption across the entire care continuum. The region's culture of early adoption and experimentation with emerging technologies, including artificial intelligence and cloud computing, further cements its position at the forefront of the global market.
Region with the Highest CAGR
Asia-Pacific is poised to hold the highest Compound Annual Growth Rate (CAGR) and is poised for the most rapid expansion, positioning it to capture a significantly larger market share in the coming years. This explosive growth is fueled by the region's massive and diverse population bases, astronomically rising smartphone and internet penetration, a wave of ambitious government digital health initiatives (such as India's National Digital Health Mission and China's "Internet+ Healthcare" strategy), and a broad-based trend toward healthcare digitization across both public and private sectors.
The region is home to over 60% of the world's diabetic population, with countries like India and China at the epicenter, creating an almost insatiable demand for efficient, scalable solutions for managing chronic disease medications. This demand is met by ePrescribing, which offers a more efficient and affordable model than traditional paper-based systems. The region also faces critical shortages of healthcare providers in rural areas, making digital tools that extend their reach essential. Growing acceptance of digital health tools among pharmacists and physicians, coupled with numerous innovative public-private partnerships in health technology, are creating a fertile environment for rapid and sustained market expansion throughout the forecast period.
By Region
North America
Europe
- Germany
- The U.K.
- France
- Italy
- Russia
- Spain
- Benelux
- Nordic
- Rest of Europe
Asia-Pacific
- China
- Japan
- South Korea
- India
- ANZ
- ASEAN
- Rest of Asia-Pacific
Latin America
- Brazil
- Mexico
- Argentina
- Colombia
- Rest of Latin America
Middle East & Africa
- Saudi Arabia
- UAE
- South Africa
- Israel
- Egypt
- Rest of MEA
Global ePrescribing Market: Competitive Landscape
The Global ePrescribing Market is characterized as moderately fragmented, featuring a dynamic and competitive environment that includes a diverse array of players. These range from giant EHR vendors for whom ePrescribing is a core module, to specialized standalone ePrescribing solution providers who focus on best-in-class functionality, to agile telemedicine platforms that build prescribing directly into their virtual care models, and pharmacy technology companies that facilitate the receiving end of the transaction. Leading established players such as Epic Systems Corporation, Cerner Corporation, Allscripts Healthcare Solutions, and Athenahealth dominate the market landscape through their deeply integrated EHR-ePrescribing offerings, which are embedded in the daily workflows of thousands of hospitals and clinics.
Cloud-based and network service specialists like DrFirst, Inc., Surescripts, and RxNT have carved out essential roles by focusing on superior interoperability, robust prescription routing services, and real-time benefit check tools that add significant value. Telemedicine leaders Teladoc Health, Amwell, and MDLive have made ePrescribing a critical, integrated component of their virtual consultation platforms. The market also features a cohort of emerging innovators who are focusing on niche areas such as AI-enhanced prescribing assistants, mobile-first solutions for independent practitioners, and the application of blockchain technology to create ultra-secure prescription protocols, particularly for controlled substances, ensuring ongoing competition and technological advancement.
Some of the prominent players in the Global ePrescribing Market are
- Epic Systems Corporation
- Cerner Corporation
- Allscripts Healthcare Solutions
- Athenahealth
- DrFirst, Inc.
- Surescripts
- RxNT
- Practice Fusion
- eClinicalWorks
- NextGen Healthcare
- Teladoc Health
- Amwell
- MDLive
- Computer Programs and Systems, Inc. (CPSI)
- Henry Schein Medical Systems
- Other Key Players
Recent Developments in the Global ePrescribing Market
- November 2025: DrFirst launches AI-powered prescribing assistant
DrFirst introduced a groundbreaking AI-driven clinical assistant that deeply integrates with existing ePrescribing workflows. This advanced tool provides real-time, evidence-based therapy recommendations, suggests context-aware dosage adjustments based on patient-specific factors like renal function, and offers predictive analytics to identify and support patients at high risk for non-adherence, thereby enhancing both the safety and personalization of prescribing.
- October 2025: Epic and Cerner demonstrate interoperable ePrescribing at HIMSS
The two leading EHR vendors jointly showcased significant advancements in interoperability for ePrescribing across their traditionally separate health system environments. The demonstrations highlighted seamless medication history exchange and reliable cross-network prescription routing, representing a major step toward overcoming one of the most significant barriers to a fully connected national medication management system.
- September 2025: Surescripts expands national record locator service
Surescripts, a key player in the U.S. ePrescribing network, announced a major expansion of its national record locator and exchange service. The enhanced service now incorporates real-time prescription benefit (RTPB) information and streamlined electronic prior authorization support, providing providers with critical cost and coverage data directly at the point of prescribing and dramatically simplifying the administrative process.
- August 2025: EU launches cross-border ePrescribing for all member states
The European Commission officially announced the full operational rollout of its cross-border ePrescribing service via the eHealth Digital Service Infrastructure (eHDSI). This landmark achievement allows citizens from any participating EU member state to have their electronic prescriptions dispensed in any other participating country, greatly facilitating safe and continuous medication access for travelers, expatriates, and cross-border workers.
- July 2025: India’s National Digital Health Mission integrates ePrescribing
In a significant move for public health digitization, India’s ambitious National Digital Health Mission (NDHM) fully integrated a standardized ePrescribing module into its national digital health stack. This enables secure electronic prescription generation and transmission across a vast network of both public and private health facilities, laying the foundation for a unified medication record for millions of citizens.
- June 2025: FDA and DEA issue joint guidance on EPCS for telehealth
In response to the permanent expansion of telehealth, U.S. regulatory agencies the Food and Drug Administration (FDA) and the Drug Enforcement Administration (DEA) issued clarified and unified guidelines for the electronic prescribing of controlled substances (EPCS) via telehealth platforms. This guidance provides the legal and technical framework needed to support the continued and safe growth of virtual care for conditions requiring controlled medications.
- April 2025: Apple Health integrates with ePrescribing platforms
Apple Inc. announced a deeper and more robust integration between its Apple Health Records app and several major ePrescribing and EHR platforms. This enhancement allows patients to automatically view, manage, and track their current medications and prescription history directly from their iPhones, empowering them with greater access to their own health information and promoting medication adherence.
Report Details
| Report Characteristics |
| Market Size (2025) |
USD 3,492.3 Mn |
| Forecast Value (2034) |
USD 17,755.1 Mn |
| CAGR (2025–2034) |
19.8% |
| The US Market Size (2025) |
USD 1,189.5 Mn |
| Historical Data |
2019 – 2024 |
| Forecast Data |
2026 – 2034 |
| Base Year |
2024 |
| Estimate Year |
2025 |
| Report Coverage |
Market Revenue Estimation, Market Dynamics, Competitive Landscape, Growth Factors and etc. |
| Segments Covered |
By Type (Integrated EHR-Based ePrescribing Systems, Standalone ePrescribing Systems, Cloud-Based ePrescribing Solutions, Web-Based/Mobile-Based ePrescribing Platforms, Others), By Application (Medication Management & Error Reduction, Electronic Prescribing of Controlled Substances (EPCS), Specialty Drug Prescribing, Telemedicine-Based Prescriptions, Refill & Renewal Management, Formulary Management & Clinical Decision Support (CDS), Prior Authorization & Compliance Management), By End User (Hospitals & Health Systems, Office-Based Physicians, Pharmacies, Telemedicine Platforms, Ambulatory Care Centers) |
| Regional Coverage |
North America – The US and Canada; Europe – Germany, The UK, France, Russia, Spain, Italy, Benelux, Nordic, & Rest of Europe; Asia-Pacific – China, Japan, South Korea, India, ANZ, ASEAN, Rest of APAC; Latin America – Brazil, Mexico, Argentina, Colombia, Rest of Latin America; Middle East & Africa – Saudi Arabia, UAE, South Africa, Turkey, Egypt, Israel, & Rest of MEA |
| Prominent Players |
Epic Systems Corporation, Cerner Corporation, Allscripts Healthcare Solutions, Athenahealth, DrFirst, Inc., Surescripts; RxNT, Practice Fusion, eClinicalWorks, NextGen Healthcare, Teladoc Health, Amwell, MDLive, Computer Programs and Systems, Inc. (CPSI), Henry Schein Medical Systems, and Other Key Players |
| Purchase Options |
We have three licenses to opt for (Single User License (Limited to 1 user), Multi-User License (Up to 5 Users), and Corporate Use License (Unlimited User) along with free report customization equivalent to 0 analyst working days, 3 analyst working days, and 5 analyst working days respectively. |
Frequently Asked Questions
The Global ePrescribing Market size is estimated to have a value of USD 3,492.3 million in 2025 and is expected to reach USD 17,755.1 million by the end of 2034.
The market is growing at a Compound Annual Growth Rate (CAGR) of 19.8 percent over the forecasted period from 2025 to 2034.
The US ePrescribing Market is projected to be valued at USD 1,189.5 million in 2025. It is expected to witness subsequent strong growth in the upcoming period, reaching USD 5,509.3million in 2034, expanding at a CAGR of 18.6%.
North America is expected to have the largest market share in the Global ePrescribing Market with a dominant share of about 40.5% in 2025.
Some of the major key players in the Global ePrescribing Market are Epic Systems Corporation, Cerner Corporation, Allscripts Healthcare Solutions, Athenahealth, DrFirst, Inc., Surescripts, and many others.