Fabry Disease treatment mainly focuses on enzyme replacement therapy and supportive care to manage symptoms and prevent complications. The growing occurrence of Fabry disease, coupled with the increasing adoption of innovative therapies like chaperone treatment, is a significant factor driving the growth of the global Fabry disease treatment market.
Moreover, extensive research and development activities in biotechnology, drug discovery, and the potential approval of promising pipeline products are expected to boost market growth further during the forecast period.
Market demand has seen an upsurge for precision medicine and personalized medicine biomarker-driven therapies tailored specifically for individual patient profiles; thus opening doors for companies developing targeted therapies to address the unmet needs of Fabry disease patients.
Research and development efforts relating to gene therapies, stem cell therapy, and regenerative medicine for Fabry disease are becoming a priority, particularly with the FDA's recent approvals of newer treatments like Migalatat, which could propel its market expansion.
Patient advocacy networks are also raising awareness and encouraging investment into treatment for this rare disorder, especially in emerging markets where diagnosis rates remain low.
Fabry Disease Treatment Market Demand has seen a significant increase, with enzyme replacement therapy (ERT) becoming the dominant therapy, accounting for around 75% of market share as of 2023.
The global market for Fabry disease therapies have expanded by an average annual increase of 12 percentage points since 2007, driven by increasing diagnoses that reached more than 10,000 in 2023 globally. Clinical trials and advanced pharmacogenomics research continue to play a major role in this expansion.
Key Takeaways
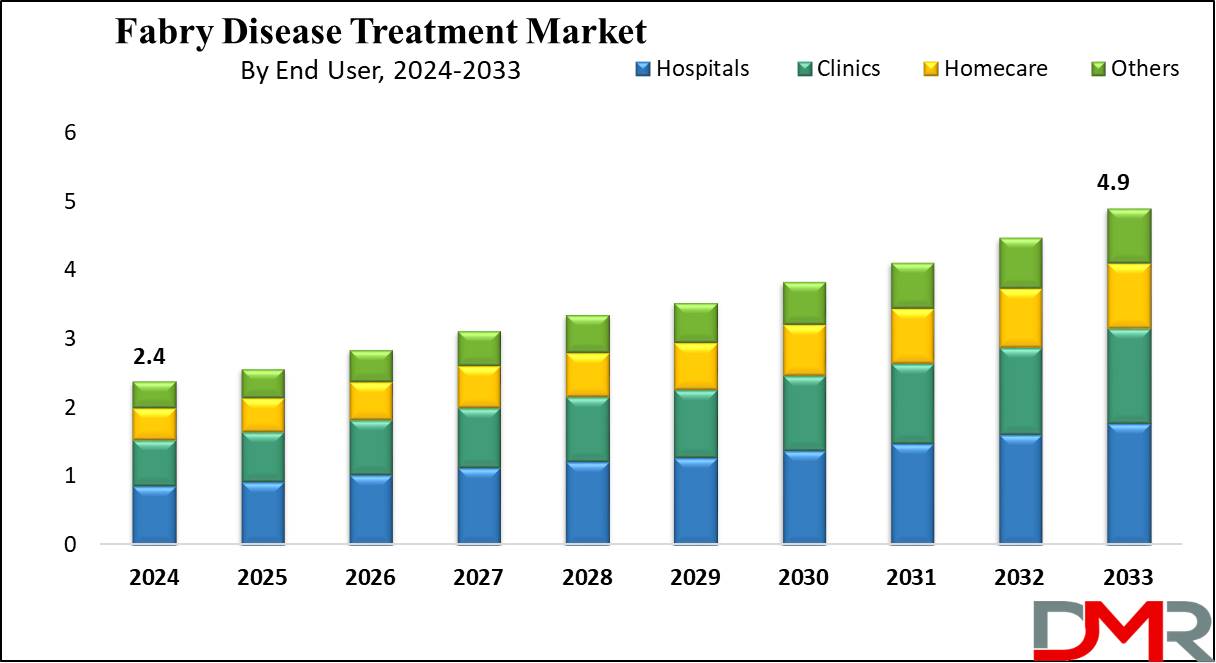
- Market Size: Global Fabry Disease Treatment Market is expected to grow by 2.3 billion, at a CAGR of 8.3 % from 2025 to 2033.
- Market Definition: Fabry disease treatment involves enzyme replacement therapy (ERT) to supplement deficient enzymes, which also includes oral chaperone therapy for specific genetic mutations.
- Treatment Analysis: Enzyme Replacement Therapy (ERT) is predicted to dominate in the Global Fabry Disease Treatment Market as it holds the market revenue share of 31.5 % based on treatment in 2024.
- Route of Administration Segment: Oral is predicted to dominate in the context of the route of administration in the global market as it holds the highest revenue market share in 2024.
- End users Analysis: Hospital are predicted to dominate the global market based on end-users as they hold the largest market share of 36.0% in 2024.
- Regional Analysis: North America is expected to dominate the global Fabry Disease Treatment market as it holds 34.1 % of the market share in 2024.
Use Cases
- Enzyme Replacement Therapy: ERT is used to treat Fabry disease which involves regular intravenous infusions of a synthetic version of the missing enzyme, that helps reduce the accumulation of globotriaosylceramide (Gb3) in various tissues.
- Gene Therapy: This treatment aims to provide a long-term solution by correcting the underlying genetic defect which allows patients' bodies to produce the enzyme endogenously, potentially offering a one-time treatment that could alleviate symptoms & prevent disease progression.
- Pain Management: This treatment helps in managing the chronic pain associated with Fabry disease and is important for improving patients' quality of life.
- Chaperone Therapy: It is a treatment approach that involves small molecules designed to stabilize the dysfunctional alpha-galactosidase A enzyme, enhancing its activity.
Market Dynamic
Drivers
Rising Prevalence of Fabry Disease Treatment
The global Fabry disease market is expected to expand due to the increasing cases of rare genetic diseases. Market research indicates that Fabry disease affects between 1 in 1,000 to 1 in 9,000 people. There is a greater chance of passing hereditary conditions to newborns due to the global rise in population. This condition impacts individuals of all ethnicities and genders, broadening the demographic of patients with Fabry disease.
Innovation in Diagnostic Technologies
Fabry disease is known to be underdiagnosed due to the lack of precise diagnostic tools. However, the Medical and healthcare sectors have made accurate and efficient diagnosis possible due to recent technological advancements. These are expected to drive the market growth rate further, ensuring better outcomes for Fabry disease patients and boosting the market growth.
Restraints
Lack of Cure against Fabry disease
Fabry disease treatment market growth is hindered due to the absence of a definitive cure for this disease. The healthcare industry is currently focused on managing the symptoms of Fabry disease and alleviating the associated pain. Furthermore, the high cost associated with its long-term treatment limits the number of patients who can afford the treatment, thereby restricting the Fabry disease treatment market size.
Inadequate Healthcare Infrastructure
This market faces significant challenges due to inadequate healthcare infrastructure and lack of effective healthcare programs, particularly in developing and underdeveloped countries, with a large portion of the population unable to access primary medical care. Also, shortage of skilled medical professionals well-versed in the management of Fabry disease creating additional obstruction to the growth.
Opportunities
Increasing Approval of New Treatment Therapies
Consistent research into the increasing prevalence of Fabry disease and its symptoms, along with investments in developing novel therapies, is expected to present significant growth opportunities for the Fabry disease treatment industry. Many
pharmaceutical companies and healthcare agencies are working towards creating effective treatment plans and improved diagnostic tools as the condition affects a large portion of the population, which accelerates Fabry disease market growth.
Rising Healthcare Investment
There is global demand for quality healthcare which is driven by an increase in healthcare expenditure, with regional governments and international agencies striving to create an accessible healthcare ecosystem. This increase in healthcare investment is likely to open new avenues for growth, as indicated by comprehensive Fabry disease treatment market research and market analysis.
Trend
Rising Investment in R&D
There is increasing investment & innovation in the R&D of novel and effective therapies for Fabry disease, which are creating significant trends for the Fabry disease treatment market. Emerging approaches like gene therapy, stem cell therapy, and gene editing are being explored to correct genetic defects or restore enzyme production in Fabry disease patients. These innovative therapies hold the potential to provide a cure or long-term solution for Fabry's disease.
Research Scope and Analysis
By Treatment
Enzyme Replacement Therapy (ERT) is expected to dominate the Fabry disease treatment market with the largest market share of 31.5 % based on the treatment in 2024. These treatments including Agalsidase Beta & Agalsidase Alfa, target the root cause of the disease by mimicking alpha-galactosidase actions, contributing to substantial clinical benefits. Fabrazyme is another therapy contributing to increased demand due to its focus on addressing the underlying cause of the disease. This segment is expected to expand significantly, by providing missing or malfunctioning enzymes to address various health problems caused by genetic disorders.
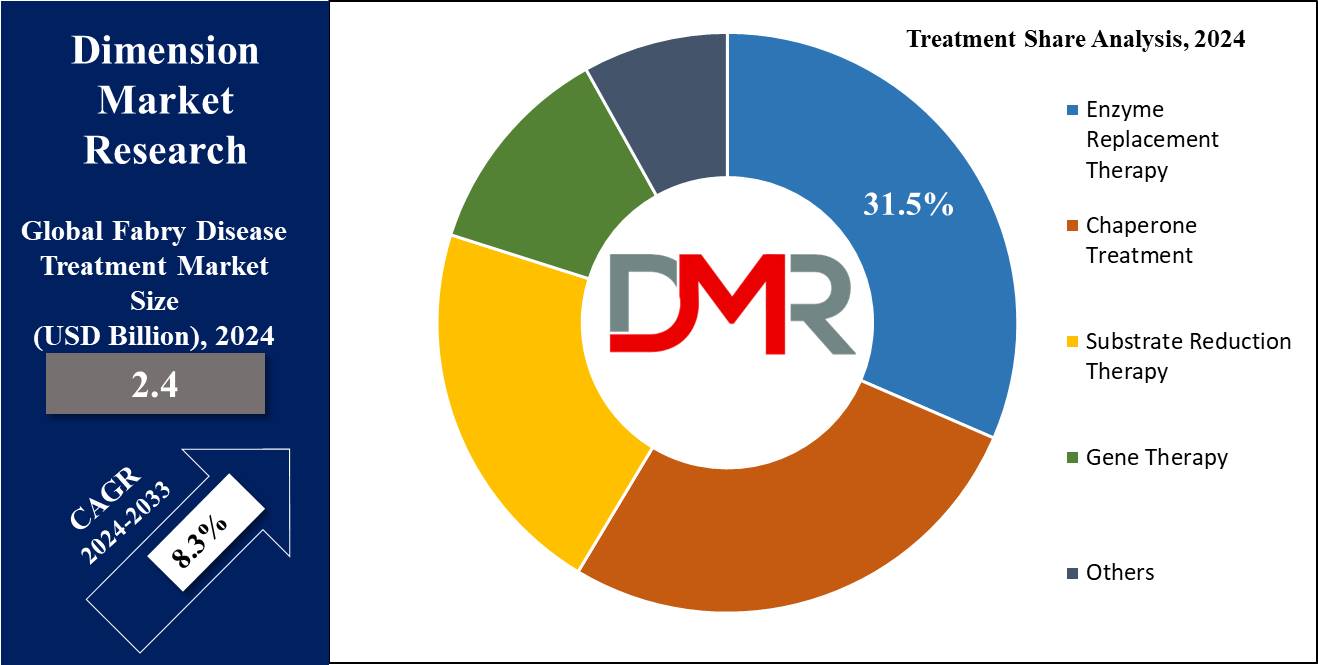
Further, Chaperon treatment is used significantly for Fabry disease, targeting the specific genetic mutations in the GLA gene. These mutations result in the production of a partially functional AGAL enzyme that is unable to properly degrade Gb3. This treatment involves the administration of small molecules or compounds, often in the form of oral medication, that act as chaperones for the partially functional AGAL enzyme. It stabilizes the enzyme's structure, improves its folding, & enhances its transport to the lysosomes, where it can effectively degrade Gb3.
By Route of Administration
Oral route is predicted to dominate the Fabry Disease Treatment Market based on the route of administration with the maximum revenue share in 2024. They offer a convenient way of administering treatments, which is particularly advantageous for patients with chronic conditions like Fabry disease as they require long-term therapy. These routes of administration eliminate the requirement of injection which is uncomfortable for many patients, especially those requiring frequent and continuous treatments. It reduces the anxiety of patients & improves the overall treatment experience, contributing to better patient satisfaction & treatment continuation.
It also offers flexibility in dosing schedules, allowing for personalized treatment plans according to individual patient needs. It is self-administered at home which reduces the burden on healthcare facilities & resources associated with hospital or clinic-based treatments. On the other hand, parenteral administration is also gaining momentum as it includes intravenous & subcutaneous injections. It ensures direct & rapid delivery of medications into the bloodstream, often preferred for Fabry Disease Treatment.
By End User
Hospital is anticipated to dominate the Fabry disease treatment market with the largest revenue share of 36.0% based on the end user in 2024 and is anticipated to experience significant growth in the forecasted period. They are known for administering enzyme replacement therapy (ERT), which is the standard treatment for Fabry disease. It involves injecting synthetic enzymes into the bloodstream to compensate for the missing or faulty ones.
One of the most important things about ERT is that it improves symptoms & the quality of life for Fabry disease patients, it doesn't cure the underlying genetic disorder. Thus, patients receiving ERT must continue it indefinitely or until a more effective treatment emerges which costly and resource-intensive procedure requiring specialized manufacturing, storage, and delivery, with hospitals bearing most of these responsibilities and expenses, expected to drive market growth.
Clinics play an important in fabry disease treatment by offering specialized & comprehensive care for this rare and complex condition. They provide diagnostic services, including
genetic testing, to confirm the disease and identify specific mutations in the GLA gene. This information helps to determine the most suitable treatment and assesses the risk of inheritance among family members.
Global Fabry Disease Treatment Market Report is segmented based on the following
By Treatment
- Enzyme Replacement Therapy
- Chaperone Treatment
- Substrate Reduction Therapy
- Gene Therapy
- Others
By Route of Administration
By End User
- Hospitals
- Clinics
- Homecare
- Others
Regional Analysis
North America is expected to dominate the Fabry disease treatment market with a revenue share of
34.1% in 2024. The healthcare system in this region is sorted using genetic screening and
molecular diagnostics, which makes identifying the disease in symptomatic individuals and asymptomatic carriers easier, which drives the growth of this market.
This region shows significant improvement in diagnostic capabilities as the disease was often misdiagnosed or remained undiagnosed due to its variable & nonspecific symptoms earlier. Further, the development of innovative treatments in this region not only reduces symptoms but also slows the progression of the disease, enhancing the overall quality of life for Fabry disease patients.
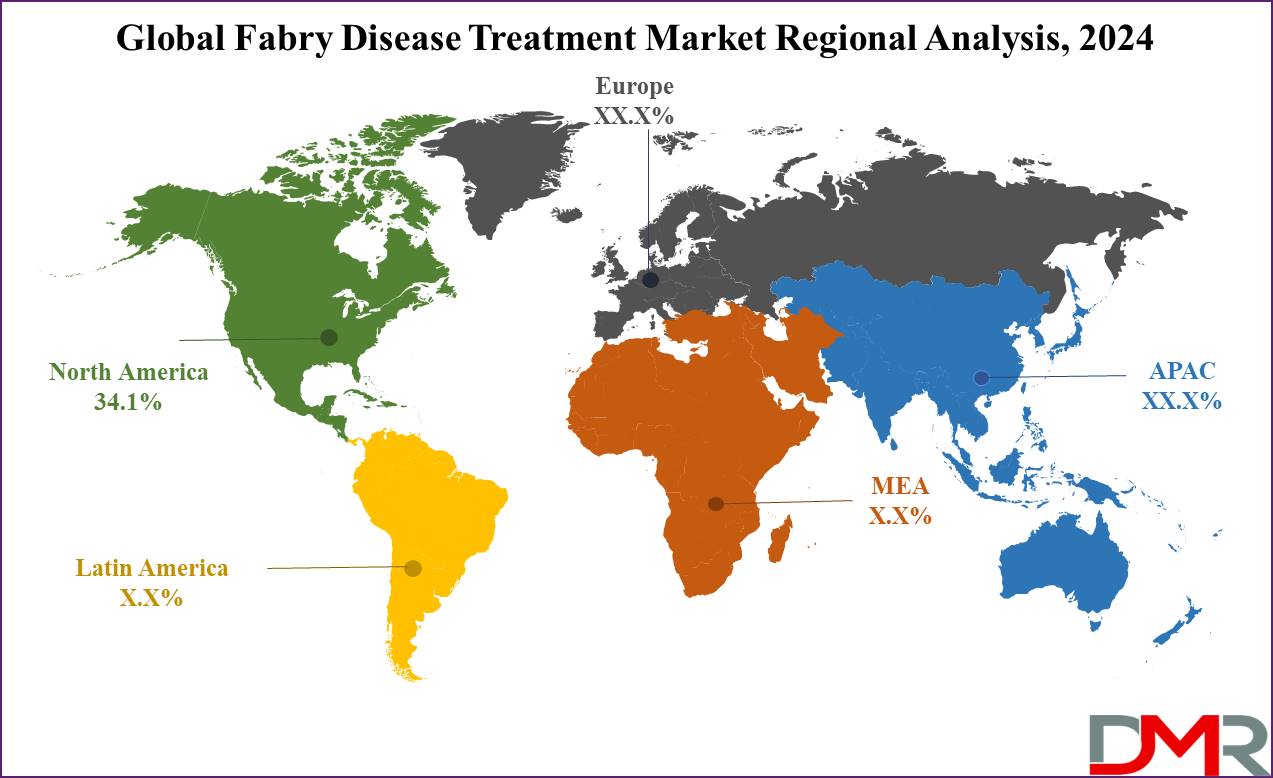
Meanwhile, Asia Pacific is the second-largest growing region for the Fabry disease treatment market over the forecast period. The market is expected to witness a surge in this region due to the geriatric population, the development of a broad range of Fabry disease treatments, and improved accessibility to pharmaceutical products. Significant advancements and improvements in Fabry disease treatment in the region further contribute to this growth.
By Region
North America
Europe
- Germany
- The U.K.
- France
- Italy
- Russia
- Spain
- Benelux
- Nordic
- Rest of Europe
Asia-Pacific
- China
- Japan
- South Korea
- India
- ANZ
- ASEAN
- Rest of Asia-Pacific
Latin America
- Brazil
- Mexico
- Argentina
- Colombia
- Rest of Latin America
Middle East & Africa
- Saudi Arabia
- UAE
- South Africa
- Israel
- Egypt
- Rest of MEA
Competitive Landscape
Key players in the fabry disease treatment market are evaluated based on their product & service offerings, financial statements, key developments, strategic approach to the market, position in the market, geographical penetration, and other key features. The market is expected to witness significant growth, driven by the contributions of these key market players and their strategic initiatives. This comprehensive analysis highlights how these players operating in the Fabry disease market are boosting market growth through innovation and strategic positioning. Companies in the market are investing heavily in research and development to diversify their product offerings, aiming to further expand the Hearth market.
Manufacturers are also engaged in various strategic initiatives to enhance their global presence. Major developments include the introduction of new products, strategic partnerships and collaborations, mergers and acquisitions, increased investments, and alliances with other organizations.
Some of the prominent players in the Global Fabry Disease Treatment Market are
- Sanofi Genzyme
- Takeda Pharmaceutical Company Limited
- Amicus Therapeutics, Inc.
- JCR Pharmaceuticals Co., Ltd.
- Idorsia Pharmaceuticals Ltd.
- Protalix BioTherapeutics, Inc.
- AVROBIO, Inc.
- Freeline Therapeutics Holdings plc
- Green Cross Corp.
- Pfizer, Inc
- Others
Recent Development
- In May 2023, Chiesi Global Rare Diseases and Protalix BioTherapeutics, Inc. obtained FDA approval for Elfabrio (pegunigalsidase alfa-ix) in the United States to treat adult patients with Fabry disease which is available as a preservative-free solution in a single-dose vial.
- In May 2023, Sangamo Therapeutics, Inc., a genomic medicine company, was granted Fast Track Designation by the FDA for their gene therapy product candidate, isaralgagene civaparvovec (ST-920), aimed at treating Fabry disease.
- In May 2023, Chiesi Farmaceutici and Protalix BioTherapeutics received approval to market their product PRX-102 in Europe for the treatment of Fabry disease.
- In September 2022, the Orphan Drug Designation (ODD) was granted to AL01211, developed by AceLink Therapeutics, for treating Fabry disease, demonstrated high potency and is recognized as a much-needed oral treatment option compared to other available therapies.
Report Details
|
Report Characteristics
|
| Market Size (2024) |
USD 2.4 Bn |
| Forecast Value (2033) |
USD 4.9 Bn |
| CAGR (2024-2033) |
8.3% |
| Historical Data |
2018 – 2023 |
| Forecast Data |
2024 – 2033 |
| Base Year |
2023 |
| Estimate Year |
2024 |
| Report Coverage |
Market Revenue Estimation, Market Dynamics, Competitive Landscape, Growth Factors and etc. |
| Segments Covered |
By Treatment (Enzyme Replacement Therapy, Chaperone Treatment, Substrate Reduction Therapy, Gene Therapy, and Others), By Route of Administration (Oral, Parenteral, and Others), By End Users (Hospitals, Clinics, Homecare, and Others) |
| Regional Coverage |
North America – The US and Canada; Europe – Germany, The UK, France, Russia, Spain, Italy, Benelux, Nordic, & Rest of Europe; Asia- Pacific– China, Japan, South Korea, India, ANZ, ASEAN, Rest of APAC; Latin America – Brazil, Mexico, Argentina, Colombia, Rest of Latin America; Middle East & Africa – Saudi Arabia, UAE, South Africa, Turkey, Egypt, Israel, & Rest of MEA |
| Prominent Players |
Sanofi Genzyme, Takeda Pharmaceutical Company Limited, Amicus Therapeutics, Inc., JCR Pharmaceuticals Co., Ltd., Idorsia Pharmaceuticals Ltd., Protalix BioTherapeutics, Inc., AVROBIO, Inc., Freeline Therapeutics Holdings plc, Green Cross Corp., Pfizer, Inc, and Others, and Other Key Players |
| Purchase Options |
HVMN Inc., Thync Global Inc., Apple Inc., Fitbit Inc., TrackmyStack, OsteoStrong, The ODIN, Thriveport LLC, Muse, Moodmetric, and Other Key Players |