It is expected that the global inflammation test kit market will experience major growth in the near future, as many cases of chronic inflammatory diseases are arising and people want to have their timely diagnosis. These kits are somewhat indispensable as they deal with the diagnosis of rheumatoid arthritis, lupus, inflammatory bowel disease, and others. As the adoption of Clinical Diagnostics rises globally, inflammation detection tools such as Rapid Test Kits are becoming more important for early assessment.
Such innovation, and more, is commonsensical for increasing accessibility to diagnostics and convenience regarding inflammation testing at home. Moreover, a surge in telemedicine services has boosted the demand for testing kits for inflammation because nowadays patients can care for their health even from home. Home-based monitoring, together with
Point of Care Diagnostics, is becoming a major driver for the market.
The inflammation test kit market is set to grow considerably at a CAGR during the forecast period, from 2024 to 2033. Major geographical regions like North America, Europe, and Asia-Pacific should be the major contributors toward the growth in the inflammation test kit market. North America had the leading market share in 2023 due to chronic diseases on the rise and increasing healthcare expenditure within this market and projected to keep its position in the upcoming year as well.
In this regard, beyond the Middle East and Africa, other potential market opportunities also emerge when improvements in the healthcare infrastructure are seen. The continuous innovations in diagnostic tools and a growing emphasis on improving patient outcomes will drive good growth in this global market in the coming years.
The inflammation test kit market has experienced substantial developments with an ever-increasing demand for home-based diagnostic solutions. Due to chronic inflammatory conditions like arthritis and cardiovascular diseases becoming more prevalent, efficient inflammation detection methods are required for efficient healthcare management. Furthermore, personalized healthcare awareness fuels their adoption. This is leading to increased use of
In Vitro Diagnostics and advanced Rapid Test Kits for quick inflammation assessment.
Technological innovations are revolutionizing the market, with companies developing user-friendly inflammation test kits with biomarkers and sensors for faster, more accurate results. AI solutions further enhance test accuracy to make inflammation testing accessible and reliable.
The COVID-19 pandemic highlighted the value of inflammation testing as a real-time way of monitoring health conditions in real time. As more patients sought remote healthcare solutions, at-home testing kits quickly gained popularity - this trend should keep demand for such kits as viable diagnostic alternatives high.
As global attention turns toward preventive healthcare, opportunities in the inflammation test kit market have expanded exponentially. Expanding healthcare access in developing regions and government initiatives that promote health screenings all contribute to market expansion. The integration of
Healthcare IT Solutions is further enhancing remote monitoring, digital diagnostics, and data-driven healthcare delivery, strengthening the adoption of inflammation testing tools worldwide. Strategic collaborations and partnerships between biotech companies and healthcare providers offer potential for innovative product development within this space.
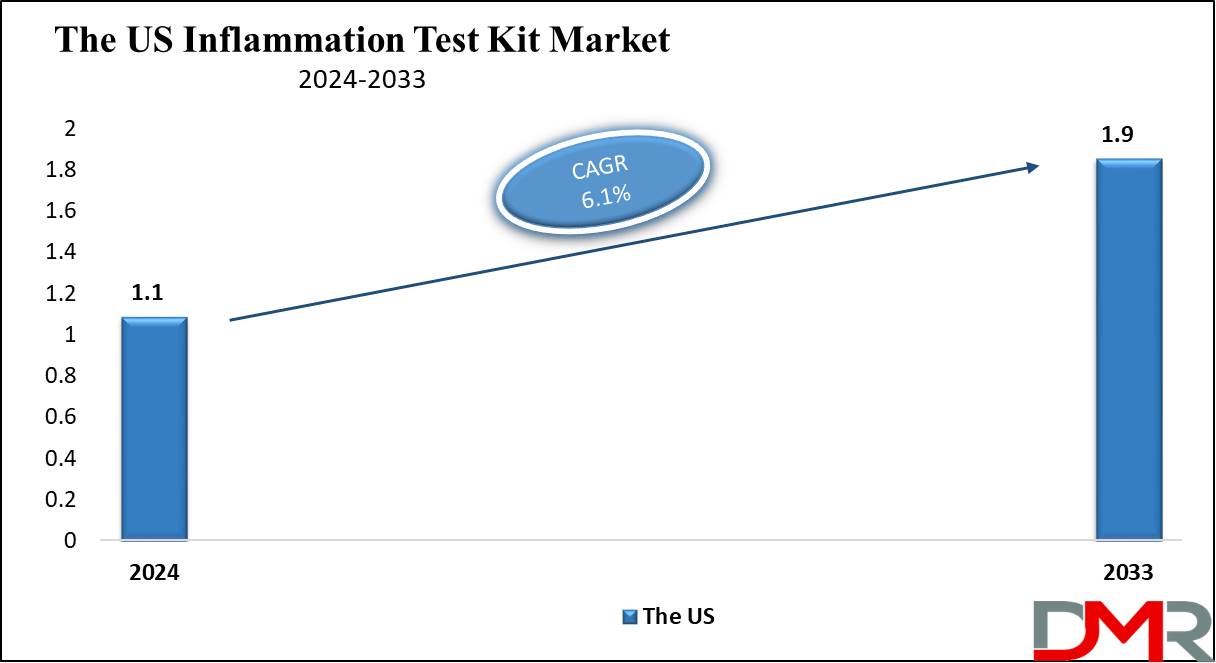
The US Inflammation Test Kit Market
The US Inflammation Test Kit Market is projected to be valued at USD 1.1 billion in 2024. It is expected to witness subsequent growth in the upcoming period as it holds USD 1.9 billion in 2033 at a CAGR of 6.1%.
Inflammation test kit sales in the U.S. have experienced exponential growth due to rising healthcare awareness and rising prevalence rates of chronic inflammatory conditions, while demand will only accelerate through 2024 as healthcare practitioners emphasize early diagnosis and personalized medicine practices. Increased dependence on Clinical Diagnostics and Point of Care Diagnostics is also contributing to higher test kit usage.
- Another key trend in the U.S. marketplace is the continued rise of home-based inflammation Another trend gaining steam in the U.S. market is home-based inflammation testing kits, enabling patients to track conditions like arthritis and cardiovascular diseases from remote monitoring at home. Companies like EverlyWell and LabCorp OnDemand recognize an opportunity in this regard and are developing more user-friendly formats at home for testing purposes.
- Technological advancements also play a large role. U.S.-based companies such as Abbott are investing heavily in research and development efforts for more precise diagnostics as a result, portable test kits designed specifically for outpatient settings like clinics are becoming available - driving market growth forward.
- With the increase of telehealth services being integrated into U.S. inflammation test kit markets, many healthcare providers now employ various platforms that enable remote diagnosis and management of inflammatory conditions resulting in higher demand for diagnostic kits.
- Intensifying government funding of healthcare initiatives combined with rising incidence rates is expected to propel market expansion over the forecast period in the US.
Key Takeaways
- Global Market Value: The global inflammation test kit market size is estimated to have a value of USD 3.3 billion in 2024 and is expected to reach USD 5.8 billion by the end of 2033.
- The US Market Value: The US inflammation test kit market is projected to be valued at USD 1.9 billion in 2033 from a base value of USD 1.1 billion in 2024 at a CAGR of 6.1%.
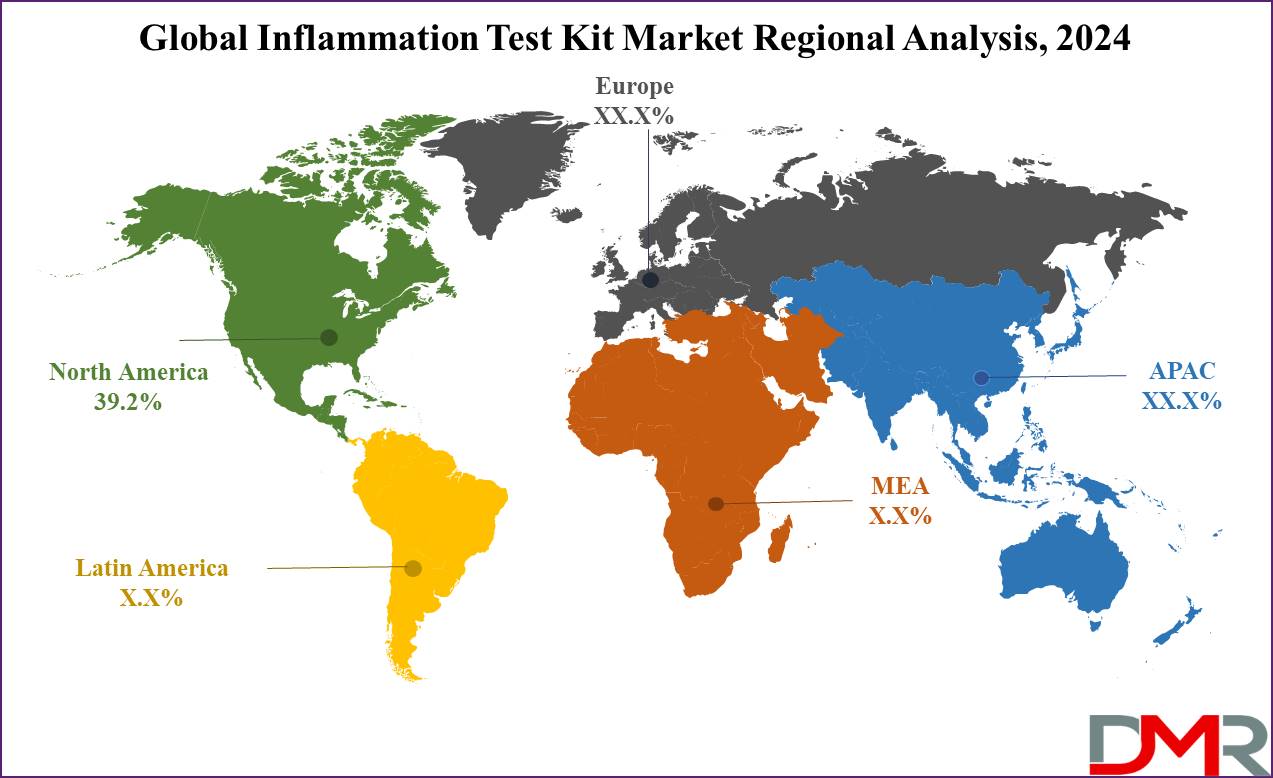
- Regional Analysis: North America is expected to have the largest market share in the Global Inflammation Test Kit Market with a share of about 39.2% in 2024.
- By Test Type Segment Analysis: Immunoassay is projected to dominate the test type segment in this market as it will hold 27.1% of the market share in 2024.
- By Application Segment Analysis: Inflammation diagnosis is anticipated to dominate the application segment in the global inflammation test kit market with 27.1% of the market share in 2024.
- Key Players: Some of the major key players in the Global Inflammation Test Kit Market are Abbott Laboratories, Roche Diagnostics, Siemens Healthineers, Thermo Fisher Scientific Inc., and many others.
- Global Growth Rate: The market is growing at a CAGR of 6.5 percent over the forecasted period.
Use Cases
- Rheumatoid Arthritis Diagnosis: Testing kits designed to assess inflammation are ideal tools in diagnosing CRP and ESR markers in those suffering from rheumatoid arthritis; thus enabling early identification for treatment to avoid long-term disability in these individuals.
- Cardiovascular Disease Risk Assessment: Inflammation test kits provide an invaluable service in the assessment of cardiovascular risk, elevated CRP levels indicate heart conditions that necessitate early diagnosis for preventative measures and more efficient management.
- Monitoring Inflammatory Bowel Disease (IBD): Testing kits can assist physicians in monitoring Crohn's and ulcerative colitis disease processes by tracking levels of inflammatory markers in their bloodstream, providing vital data that enables informed decisions for tailored treatment plans.
- COVID-19-related Inflammation: Testing kits were instrumental during the pandemic in measuring inflammation among COVID-19 patients and monitoring CRP levels to assess the risk for serious complications.
Market Dynamic
Trends
Home-based Test Kits
A key trend witnessed in the global inflammation test kit market is the growing demand for home-based healthcare solutions. Various enterprises are, therefore, developing and introducing home-based diagnostics in tune with the growing demand that are convenient and patient-friendly.
For instance, Everlywell and Vitall have introduced home-based inflammation test kits to enable patients suffering from rheumatoid arthritis and cardiovascular diseases to monitor the level of inflammation in their bodies.
These kits are gaining fame due to the convenience they carry; instead of having patients at healthcare facilities, they will collect samples and send them to the labs for testing. This trend is likely going to increase in momentum with increased technological advancements that further enhance the accuracy and ease-of-use qualities of home-based kits.
Telemedicine Integration
Telemedicine-integrated diagnosis of inflammation is revolutionizing the way patients are cared for. This is being taken to the next level through telemedicine platforms, whereby, through test kits, inflammatory conditions are remotely monitored to give patients real-time consultations and diagnosis without necessarily having to make house calls.
This trend has given birth to companies such as LabCorp OnDemand and Nebula Genomics, which are quickly taking advantage of the trend with test kits available for use in telemedicine services. This development brings a factor of convenience and accessibility, especially for patients living in remote or otherwise underserved areas, thereby likely to drive market growth in the forecast period.
Growth Drivers
Rising Prevalence of Chronic Inflammatory Diseases
Chronic inflammatory diseases, such as arthritis, cardiovascular diseases, and inflammatory bowel disease, are on the rise which acts as the major driver for the demand for test kits in the global inflammation test kits market.
As people continue to grow older, these diseases are likely to further rise, thus creating a front door for diagnostic needs capable of detecting and monitoring inflammation. Increasing awareness of the importance of diagnosis at an early stage and inflammation test kits for monitoring disease through courses enables market growth.
Technological Advancements in Diagnostics
There are significant technological advances in diagnostic tools that fuel the growth of the inflammation test kit market. Such new innovative tests include high-sensitivity immunoassays, point-of-care testing, and multiplex testing for rapid and precise detection of biomarkers of inflammation such as CRP and interleukins.
These are the very exciting reasons why inflammation test kits are increasingly becoming available in hospitals, clinics, and home-based settings, coupled with improvement in patient outcomes. Companies like CTK Biotech and Vitrosens continue to lead the way with innovative diagnostic solutions.
Growth Opportunities
Expansion in Emerging Markets
The rapid development of infrastructures in the healthcare segment in emergent geographies like Asia-Pacific, Latin America, the Middle East, and Africa has opened up enormous opportunities for the inflammation test kit market.
This region is gaining more investments in healthcare and, overall, the development of diagnostic capabilities. This, in turn, results in increased access to advanced healthcare services and rising disposable incomes. Thus, growing awareness about inflammatory diseases and their pathophysiology is driving demand for inflammation test kits. Consequently, companies that expand their presence in these major market regions will record significant growth in the next few years.
Advancements in Biomarker Research
Continuous research regarding new biomarkers for inflammation opens up new opportunities in the market for inflammation test kits. The discovery of more specific and sensitive biomarkers for a range of inflammatory diseases would allow the development of test kits that are more exacting and comprehensive in their diagnostics.
For autoimmune diseases where the early and more precise identification of inflammatory markers can improve outcomes remarkably, this is a very significant area. Key players like Abbott and Seegene develop next-generation inflammation test kits using these biomarker research advancements.
Restraints
Lack of Awareness in Developing Regions
Several incidences of chronic inflammatory diseases are increasing, and yet, lack of awareness about the inflammation test kit and its benefits in developing areas remains one of the major challenges. Most of the caregivers operating in these regions along with their patients are uninformed regarding the biomarkers' role in diagnosing as well as managing inflammatory diseases.
This represents an unmet need because of a lack of awareness among rural and underserved populations. Diagnosis, regular monitoring, and educating health care professionals and patients about the importance facilitate overcoming this barrier in the future.
Regulatory Challenges
These are the challenges posed by the stringent regulatory requirements put forward for diagnostic devices and test kits, which are a hindrance to the growth of the inflammation test kit market. New diagnostic products need to go through exhausting testing and obtain approvals from regulatory bodies such as the U.S. FDA and the European Medicines Agency.
These regulatory challenges do delay new test kit launches into the market, which offers a negative impact on innovations and subsequently on market growth. Besides this, because different regions have different standards for regulation, this also tends to complicate companies to enter multiple markets simultaneously.
Research Scope and Analysis
By Test Type
Immunoassay is projected to dominate the test type segment in the global inflammation test kit market as it will hold 27.1% of the market share by the end of 2024. Immunoassay represents the major share in the test type segment of the global inflammation test kit market due to the high accuracy, specificity, and wide range of detectable inflammatory markers. These assays have a wide range of applications in diagnostic laboratories, clinics, and hospitals for estimating biomarkers such as CRP, interleukins, and tumor necrosis factors, which are important for the diagnosis and monitoring of inflammatory diseases.
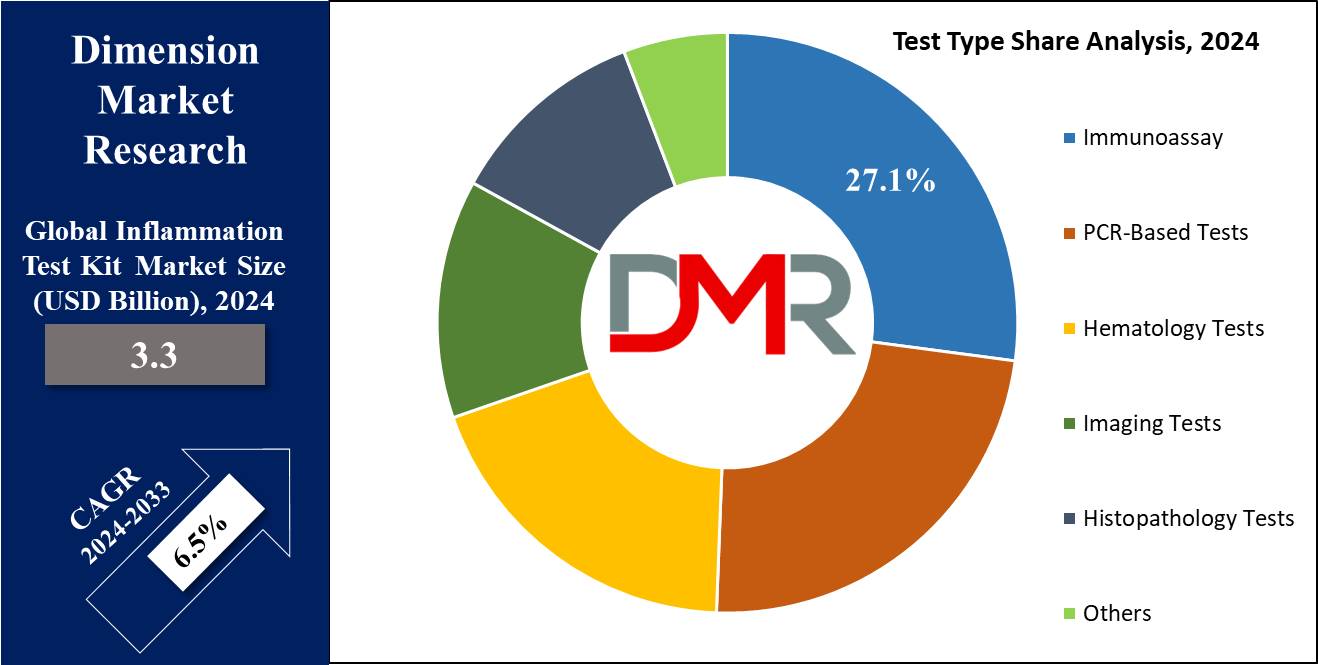
One of the main reasons for dominance in immunoassays is its ability to handle multiple samples at a time, hence highly efficient in diagnostics that involve large-scale samples. This is very important in a clinical setting, where timely and accurate results are needed for the effective management of patients. Another advantage of immunoassays involves their versatility in handling different types of samples including blood, saliva, and urine.
Other contributing factors include continuous technological advances in immunoassay techniques, including the high sensitivity and speed of ELISA and CLIA assays. In the inflammation test kit market, companies such as Abbott and Seegene which are major players are particularly innovative, keeping their competitive advantage because their products have been continuously perfected toward precision and usability.
The prevalence of chronic inflammatory diseases, along with the increasing demand for early diagnosis, has made immunoassay the test type of choice in this market. This segment is expected to continue leading the market and is projected to show a considerable share increase in the forecast period.
By Sample Type
Blood is predicted to dominate the sample type segment in the global inflammation test kit market with the highest market share in 2024. This is because blood is the best and most reliable sample type used to develop highly accurate and comprehensive findings that relate to the detection of inflammatory markers. Blood tests have thus become quite common in diagnosis, as they are a way through which biomarker levels of CRP, fibrinogen, and various pro-inflammatory cytokines could be ascertained as vital indicators of inflammation.
One of the prime reasons for the supremacy of blood samples is their capabilities in the capture of systemic inflammation, showcasing a more holistic view of the body's inflammatory response. This is especially important in diagnosing conditions like rheumatoid arthritis, cardiovascular diseases, and inflammatory bowel disease, where localized tests may not be able to give a full picture of the patient's health.
Furthermore, blood samples are easier to collect and process compared to other sample types that could be used, such as saliva or urine. Most healthcare facilities are suitably equipped to handle blood collection, and thus it is at least convenient and accessible for both the patient and the healthcare provider. In addition, blood samples can use a wide range of diagnostic technologies, which include immunoassays and molecular tests, further justifying their wide usage.
Other factors that have contributed to blood remaining the preferred sample type include the increasing need for early detection of inflammatory diseases and growing interest in personalized medicine. Technological advancements, which increase the precision and speed of blood-based tests, are expected to maintain the dominance of this sample type during the forecast period.
By Application
Inflammation diagnosis is anticipated to dominate the application segment in the global inflammation test kit market with 27.1% of the market share in 2024. The diagnostics category leads in the application segment for the global inflammation test kit market due to the surge in chronic inflammatory diseases and the growing demand for early detection and treatment. Diseases such as rheumatoid arthritis, lupus, and cardiovascular diseases will, by their nature, have diagnosis of their inflammatory markers highly critical to ensure effective management and treatment.
The main reason for early diagnosis of inflammation is due to increased awareness among the patient as well as the physician on early diagnosis of this disease. Early intervention leads to better clinical outcomes, along with a reduction in major complications. Inflammation test kits, which measure biomarkers such as CRP, interleukins, and cytokines, are vital in this respect.
Further technological advancement in diagnostic equipment has also played a significant role in the development of this application segment. The advancements in sensitivity, accuracy, and ease of use of the test kits make inflammation diagnosis increasingly feasible for various healthcare settings like hospitals, clinics, and even home-based care.
Many of the major companies in the inflation test kit market, including Abbott and LabCorp OnDemand, continuously invest in research and development to improve the precision and accuracy of the tests.
Inflammation diagnosis will remain dominant as the demand for personalized medicine widens, which will give way to applying customized treatments based on each individual's inflammatory profile. The prevalence of inflammatory conditions and continuous development in diagnostic technologies are expected to drive growth in the market share of this segment during the forecast period.
By End User
The hospitals and clinics segment is projected to dominate the end-user segment of the global inflammation test kit market, as these are key channels in the identification and management of diseases. These healthcare centers are a primary point of care for patients suffering from chronic conditions such as rheumatoid arthritis, cardiovascular diseases, and inflammatory bowel diseases, where the need for an inflammation test kit comes as a great help for diagnosis.
Because large diagnostic tests with inflammation test kits, such as measurements of CRP and ESR, need much infrastructural setup and expertise, they fall under the purview of hospitals and clinics. They have to deal with a huge number of patients, which creates an unprecedented demand for test kits that provide quick and accurate results.
Other examples of inflammation testing kits include immunoassay-based testing kits, which, being widely used in these settings, enable healthcare providers to efficiently screen and monitor patients for inflammatory conditions.
Besides this, the volume of the test samples processed in diagnostic laboratories with the aid of hospitals and clinics also adds to making the segment dominant. Full integration of the test kits within the wider diagnostic workflow ensures that hospitals and clinical tests remain key players within the inflammation test kit market.
Also, hospitals and clinics have arisen as the most important consumers of inflammation test kits, with growing diagnostic prevalence attributable to chronic diseases and increasing demand due to personal medicine. The growth in the market share will be further supported by continuous development in diagnostic technologies coupled with a rising focus on improvement in patient outcomes.
The Global Inflammation Test Kit Market Report is segmented on the basis of the following
By Test Type
- Immunoassay
- C-Reactive Protein (CRP) Test Kits
- Erythrocyte Sedimentation Rate (ESR) Test Kits
- Tumor Necrosis Factor-alpha (TNF-α) Test Kits
- Interleukin-6 (IL-6) Test Kits
- Fibrinogen Test Kits
- Procalcitonin (PCT) Test Kits
- Serum Amyloid A (SAA) Test Kits
- PCR-Based Tests
- Hematology Tests
- Imaging Tests
- Histopathology Tests
- Others
By Sample Type
- Blood
- Urine
- Stool
- Saliva
- Tissue
By Application
- Inflammation Diagnosis
- Disease Progression Monitoring
- Treatment Selection
- Treatment Efficacy Evaluation
- Inflammatory Risk factor Screening
By End User
- Hospitals & Clinics
- Diagnostic Laboratories
- Academic & Research Institutes
- Home Care Settings
Regional Analysis
North America is projected to dominate the global inflammation test kit market as it commands over
39.2% of the revenue share by the end of 2024. The leading position in the global inflammation test kit market is contributed by North America, as a highly established healthcare infrastructure, a high prevalence of chronic inflammatory diseases, and a strong focus on research and development bring momentum into the industry. Major players operating in the inflammation test kit market include Abbott and LabCorp are continuously working toward developing advanced diagnostic tools.
The incidence of various chronic conditions, such as rheumatoid arthritis, cardiovascular diseases, and diabetes, has led to strong demand in North America. This holds more in the case of the U.S., where the early diagnosis and use of personalized medicines are encouraged for better results amongst patients.
The region also involves a high level of awareness related to healthcare access to advanced diagnostic technologies. Apart from the high demand for diagnosis tools, the stringent regulatory framework in North America and government support given for healthcare initiatives very strongly drive the dominance of the market.
The region also excels in the integration of telemedicine services, thereby enhancing access to test kits for inflammation. Companies in North America are crucially investing in research and development to have more accurate, quicker, and cheaper inflammation test kits.
This guarantees the continuity of the leading position in this region. Hence, technological advancement, high healthcare expenditure, and more focus on patient care will make North America the topmost region in the global inflammation test kit market.
By Region
North America
Europe
- Germany
- The U.K.
- France
- Italy
- Russia
- Spain
- Benelux
- Nordic
- Rest of Europe
Asia-Pacific
- China
- Japan
- South Korea
- India
- ANZ
- ASEAN
- Rest of Asia-Pacific
Latin America
- Brazil
- Mexico
- Argentina
- Colombia
- Rest of Latin America
Middle East & Africa
- Saudi Arabia
- UAE
- South Africa
- Israel
- Egypt
- Rest of MEA
Competitive Landscape
The global inflammation test kit market is highly competitive, with key players developing and expanding their product portfolios continuously. Some major players operating in the market are Abbott, Everlywell, CTK Biotech, and Seegene. These companies actively contribute to improving diagnostic technologies and increasing the supply of test kits globally.
Abbott is considered one of the world leaders in inflammation testing kits. It provides high-quality, immunoassay-based products to ensure accuracy and speed of results. In view of such a trend, the company focuses on research and development through the continuous launching of new products.
It has been one of the fastest movers in the market, as Everlywell provided home testing solutions, which enabled consumers to keep up with inflammation continuously right from the comfort of their homes. The business has witnessed huge growth within USP market precincts. Precipitating major players further are CTK Biotech and Seegene, both working on projects involving advanced diagnostic tool development for clinical and home usage.
These also increase their presence within emerging regions such as the Asia-Pacific and Middle East where the demand for inflammation test kits is expanding. Overall, the competitive landscape is characterized by continuous innovation, strategic partnership, and a strong focus on the improvement of patient outcomes by early and accurate inflammation diagnosis.
Some of the prominent players in the Global Inflammation Test Kit Market are
- Abbott Laboratories
- Roche Diagnostics
- Siemens Healthineers
- Thermo Fisher Scientific Inc.
- Beckman Coulter (Danaher Corporation)
- BD (Becton, Dickinson and Company)
- bioMérieux SA
- Ortho Clinical Diagnostics
- Hologic, Inc.
- Cepheid (Danaher Corporation)
- F. Hoffmann-La Roche Ltd
- Luminex Corporation
- Quest Diagnostics Incorporated
- Other Key Players
Recent Developments
- August 2024: Abbott launched an advanced CRP inflammation test kit with improved sensitivity and faster turnaround times. This innovation is aimed at enhancing the early diagnosis of cardiovascular diseases by detecting inflammation-related biomarkers with greater accuracy.
- June 2024: Everlywell expanded its range of at-home diagnostic solutions by launching an inflammation test kit that includes comprehensive markers such as CRP and interleukins. The kit is designed to cater to individuals who prefer remote monitoring and personalized healthcare solutions.
- March 2024: CTK Biotech introduced a rapid diagnostic test kit for inflammation, specifically designed for point-of-care settings. This new product allows for faster and more efficient diagnosis of inflammatory diseases, improving patient outcomes in both hospital and outpatient settings.
- December 2023: Seegene announced a strategic partnership with leading healthcare providers in the Asia-Pacific region to roll out their inflammation test kits. The collaboration aims to improve access to high-quality diagnostics and expand Seegene’s market presence in key emerging markets.
- October 2023: LabCorp OnDemand integrated inflammation test kits into their telemedicine platform, allowing patients to monitor inflammatory markers remotely. This development is part of LabCorp’s broader effort to enhance patient access to diagnostic tools through digital health services.
- September 2023: Vitrosens launched a new multiplex test kit for detecting multiple inflammatory biomarkers simultaneously. This test is designed to be used in hospital labs, offering healthcare providers a more comprehensive view of a patient's inflammatory profile.
- July 2023: Avellino Labs expanded its diagnostic portfolio by acquiring Stablelab, a company specializing in inflammation test kits for veterinary applications. This acquisition allows Avellino to enter a new market segment and diversify its offerings.