Market Overview
Global Companion Diagnostics Market size was valued USD 8.0 billion in 2023 which is further expected to reach a market value of USD 26.0 billion in 2033 at a CAGR of 12.5%.
A companion diagnostic is a type of test designed to ensure the safe & effective use of a particular biological product or drug. They are used in cancer diagnosis to identify biomarkers to recommend the most appropriate drug, which allows for personalized treatment according to the specific condition of each patient. The global companion diagnostics market refers to the market in the healthcare industry, which is primarily centered on diagnostic methods utilized by patients affected by numerous medical conditions.
Diagnostic testing is designed to assist healthcare experts in making informed choices approximately suitable treatment solutions for male or female patients’ desires. In addition, complementary diagnostics are frequently used to perceive specific biomarkers or genetic mutations that purpose the affected clinical situation.
By studying these biomarkers, healthcare professionals determine whether this precise drug or treatment would be effective for patients with uncommon conditions. This individualized method of figuring out an affected person’s situation permits for greater targeted and advanced treatments, lowering the risk for the drug’s effects and enhancing the affected person's treatment condition. In fields like
cancer diagnostics, this targeted approach can significantly improve patient outcomes.
The global companion diagnostic market exhibits several dynamic characteristics that reflect the ongoing changes, opportunities, and challenges within the companion diagnostics market.
Key Takeaways
- Market Size: Global Companion Diagnostics Market is expected to grow by 16.0 billion, at a CAGR of 12.5 % from 2025 to 2033.
- Market Definition: Companion Diagnostics are tests used with specific medical treatments, which help doctors determine whether a particular treatment or therapy is suitable for a patient.
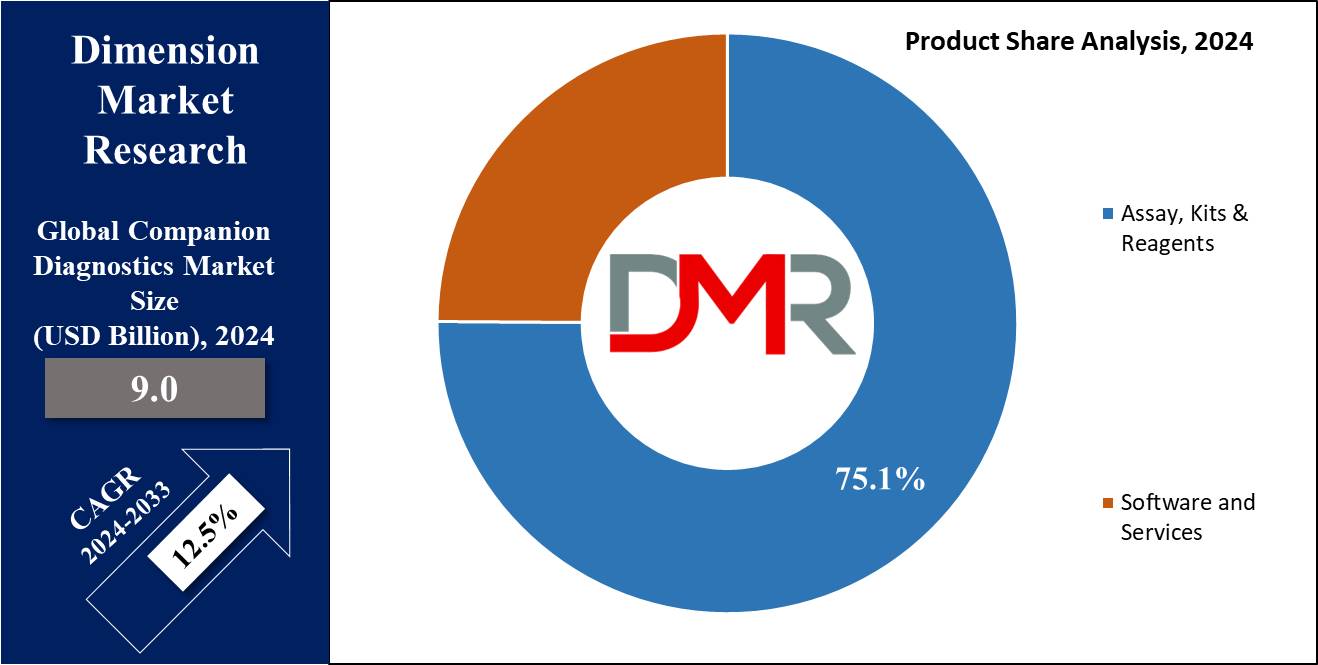
- Product Analysis: Assay, Kits & Reagents is predicted to dominate in the Global Companion Diagnostics Market as it holds the maximum revenue share of 75.1% based on products in 2024.
- Technology Segment: Polymerase chain reaction is predicted to dominate based on technology in the global market, as it holds the highest revenue market share of 38.9% in 2024.
- Indication analysis: Cancer is anticipated to emerge as the dominant force based on indication in the global market, with the largest market share in 2024.
- Sample type Review: The companion diagnostics market is divided into tissue samples, blood samples, and others. Tissue samples are predicted to dominate the global market based on the sample segment, as they hold the maximum market share in 2024.
- End users Analysis: Pharmaceutical & biopharmaceutical Companies are predicted to dominate the global market based on end-users as they hold largest of the market share in 2024.
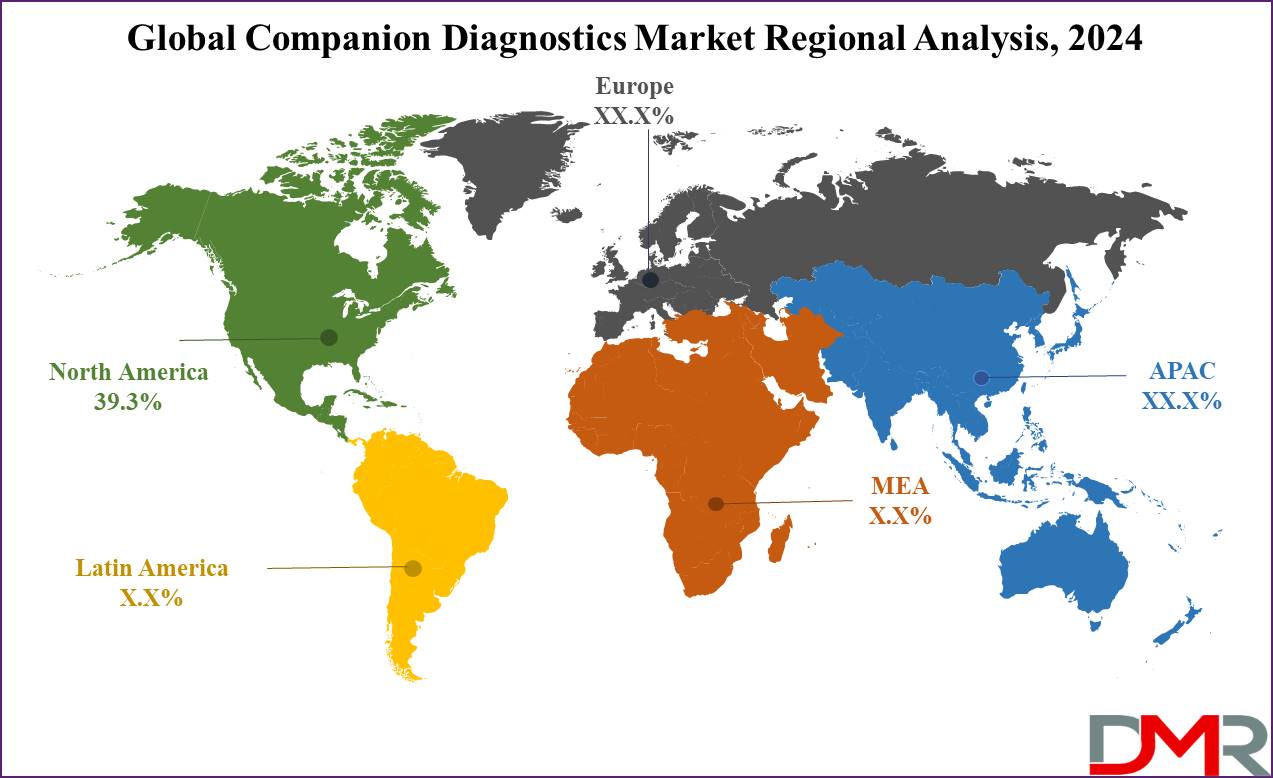
- Regional Analysis: North America is expected to dominate the global companion diagnostics market as it holds 39.3% of the market share in 2024.
Use Cases
- Infectious Diseases: Companion diagnostics is used in infectious diseases to identify genetic markers of drug resistance, which determine the most appropriate antiviral or antibiotic treatment.
- Neurological Disorders: Demand for companion diagnostics is increasing in neurology to assist in the diagnosis and treatment of conditions such as Alzheimer's disease, Parkinson's disease, and epilepsy.
- Fertility and Reproductive Health: Adoption of companion diagnostics is helpful to assess genetic factors that may affect fertility, pregnancy outcomes, or the risk of hereditary disorders in offspring, as it can identify carrier status for genetic conditions like cystic fibrosis, hemophilia, or thalassemia, allowing couples to make informed decisions.
- Cancer Treatment: There is a huge utilization of companion diagnostics in oncology to match cancer patients with the most effective targeted therapies, companion diagnostics products, or immunotherapies.
Market Dynamic
Drivers
Technological Advancement in Healthcare
Companion diagnostic market is fueled by ongoing technological progress, particularly in genomics, sequencing technologies, & diagnostic tools which leads to the development of more precise & efficient diagnostic tests, which expanded the growth of this market. Also, the integration of
artificial intelligence and machine learning into diagnostic processes is transforming the growth of this field, which provides deeper insights and faster results that support the growth of the companion diagnostic market.
Shift Towards Personalized Medicine
This market is witnessing the shift towards personalized medicine as it is gaining more recognition in the healthcare industry, which acts as a significant driver of the companion diagnostic market. Physicians and patients are now looking for treatment plans that are specifically tailored according to the individual's genetic profile and companion diagnostics completely align with this requirement, which further pushes the growth of this market.
Restraints
Financial Barriers and High Attrition Rates in Biomarker Development
High investment is required for the discovery, development, and validation of biomarkers as there is a high attrition rate of drugs during clinical trials, with nearly 30% failing in the phase III stage, which creates substantial financial obstacles for diagnostic manufacturers. Conducting diagnostic trials and meeting stringent regulatory standards demands considerable investment, which makes it difficult for smaller companies to innovate & develop new biomarkers, thus, restraining the growth of this market.
Complex and technical process
The companion diagnostic process is a complex technical process that requires trained & skilled professionals for accurate execution. There is also a lack of awareness among healthcare professionals regarding the latest advancements in companion diagnostic tests, leading to a gap in operational knowledge within clinical laboratories, which obstructs the adoption of these diagnostics and restraints the growth of this market.
Opportunities
Increasing Integration of Companion Diagnostics in Clinical Trials
The integration of companion diagnostics into clinical trials is becoming more prevalent, as leading pharmaceutical companies utilize these tests to select suitable patient populations for drug trials and evaluate treatment efficacy. This approach helps in the introduction of new drugs & treatment plans to the healthcare market, which creates more opportunities for the growth of this market.
Regulatory and Reimbursement Landscape
Regulatory bodies, such as the FDA, are establishing more defined and streamlined processes for the approval of companion diagnostics. This smooth process of regulatory bodies increased investment from companies that are eager to develop these essential tests. More clarity in the regulatory environment process reduces the uncertainty & time associated with bringing new companion diagnostics to market, which in turn accelerates innovation and availability.
Trend
Collaborations and Partnerships
There are increasing collaborations between pharmaceutical companies & diagnostic firms to co-develop companion diagnostics alongside new drugs. Governments & non-profit organizations are also partnering with private companies to support research and development in companion diagnostics.
Research Scope and Analysis
By Product
Under the segmentation, by product, the companion diagnostics market is fragmented into Assay, Kits & Reagents, and Software & Services. Assay, Kits & Reagents is predicted to lead in the global market, accounting for 75.1% of the market share in 2024, and is anticipated to show the most rapid CAGR in the upcoming years of 2024 to 2033.
The rise in the demand for assays, kits, and reagents can be attributed to various factors, as it offers comprehensive solutions for conducting diagnostic tests. These regularly are available in packaged kits that consist of all the essential components, inclusive of primers, probes, enzymes, and buffers, making it less complicated for researchers and healthcare experts to perform the tests without having to source out every individual aspect one by one.
Assays, kits, and reagents are the tangible and crucial components of partner diagnostic assessments as they allow the detection and analysis of biomarkers and genetic mutations within the diagnostic objectives. These are evolved and synthetic at a high diploma of standardization and excellent control which ensures the consistency and reliability of the diagnostics take a look at results, which is critical in medical diagnostics.
By Technology
Companion Diagnostic Market is categorized into polymerase chain reaction, Real-time PCR, situ hybridization, Immunohistochemistry, Gene Sequencing, and Others. Based on technology used in the global market, polymerase chain reaction (PCR) is anticipated to dominate this segment as it
holds 38.9% of the market share in 2024 and is anticipated to show subsequent growth in the forthcoming period.
PCR is a highly versatile technique that is widely used in molecular and cell biology. It is used in various applications, such as the detection of specific DNA or RNA sequences where it making it identifying genetic mutations and biomarkers associated with specific diseases. It is known for its high sensitivity & accuracy, which allow the detection of even small quantities of nucleic acid targets. This sensitivity is crucial in identifying low-abundance mutations or genetic variations that may be relevant for personalized medicine.
Polymerase chain reaction is more popular than other techniques as it provides relatively faster results than, as in polymerase chain reaction, the test results can be obtained in a matter of hours. As time is crucial in making timely clinical decisions the use of PCR in companion diagnostics is more popular. Polymerase chain reaction has a standard protocol for testing, which ensures the reliability of results in companion diagnostics firms.
By Indication
In terms of indication, cancer is predicted to dominate the companion diagnostic market as it holds the highest market share in 2024 and is anticipated to show the respective growth rate in the upcoming years.
The reason cancer dominates this segment is that it is one of the leading causes of death, and the growing number of cancer patients is creating a significant market for companion diagnostics, as these tests are particularly valuable in cancer management.
Cancer has a distinctly heterogeneous nature, as exclusive sorts of cancers have their own precise genetic and molecular profiles, which helps healthcare specialists make personalized remedies for the affected person according to their condition.
Companion diagnostics also can assist in figuring out precise biomarkers and mutations inside a patient's tumor, allowing oncologists to select the maximum appropriate targeted therapies for their remedy.
While cancer dominates the accomplice diagnostic market, it's vital to observe that associate diagnostics additionally keep full-size promise in other sickness areas, inclusive of neurological illnesses and infectious illnesses. As the basic information of the molecular and genetic basis of various forms of most cancers expands, greater targeted cures will expand. These factors collectively boost the growth of this segment in the global companion diagnostics market.
By Sample Type
The companion diagnostic market is segmented into tissue samples, blood samples, and other types of samples. The tissue sample is expected to dominate the companion diagnostic market with a maximum revenue share in 2024, due to their ability to directly analyze tumors, offering a thorough assessment of genetic mutations and biomarkers that facilitate precise and personalized treatment choices.
Advancements in techniques like next-generation sequencing, immunohistochemistry, and molecular diagnostics next-generation sequencing, increase the demand for tissue segments in the companion diagnostic market.
The rising occurrence of long-term diseases, including cancer, autoimmune disorders, and infectious diseases, creates a significant opportunity for this market. Tissue sample plays an important role in diagnosing and monitoring these conditions, as they provide valuable insights into the genetic and molecular characteristics of the disease, which drives the growth in this segment.
By End User
Pharmaceutical & Biopharmaceutical Companies are expected to dominate the companion diagnostic market with the largest revenue share in 2024. This dominance is due to the increasing focus on personalized medicine, which leads to the adoption of pharmaceutical companies to develop targeted therapies that require companion diagnostics for patient stratification & treatment optimization.
Also, a collaboration between pharmaceutical firms & diagnostic companies offers the integration of companion diagnostics into drug development pipelines, further boosting market growth. Regulatory agencies focus on companion diagnostics for certain drug approvals, which encourages pharmaceutical companies to invest in this segment, contributing to their dominance in the global companion diagnostic market.
The Companion Diagnostics Market Report is segmented based on the following:
By Product
- Assay, Kits & Reagents
- Software & Services
By Technology
- Polymerase Chain Reaction (PCR)
- Real-time PCR (RT-PCR)
- In-situ Hybridization (ISH)
- Immunohistochemistry (IHC)
- Gene Sequencing
- Others
By Indication
- Cancer
- Neurological disease
- Infectious Disease
- Other
By Sample Type
- Tissue Samples
- Blood Samples
- Others
By End User
- Pharmaceutical & Biopharmaceutical Companies
- Reference Laboratories
- Contract Research Organizations
- Others
Regional Analysis
North America is predicted to dominate the global companion diagnostic market as it accounts for 39.3% of the market share in 2024. The reason why this region dominates this market is because it is home to many major pharmaceutical biotechnology companies, which can actively participate in pharmaceuticals & often collaborate with research institutions to conduct partner research.
The strong presence of these pharmaceutical companies in this segment contributes significantly to the lead in the global companion diagnostics market. The segment also has a well-established regulatory framework for allied diagnostic markets such as the US. This region has many regulating bodies, like the Food & Drug Administration, which provides guidelines for the production of drugs, making it easier for pharmaceutical and diagnostic companies to bring their products to market.
By Region
North America
Europe
- Germany
- The U.K.
- France
- Italy
- Russia
- Spain
- Benelux
- Nordic
- Rest of Europe
Asia-Pacific
- China
- Japan
- South Korea
- India
- ANZ
- ASEAN
- Rest of Asia-Pacific
Latin America
- Brazil
- Mexico
- Argentina
- Colombia
- Rest of Latin America
Middle East & Africa
- Saudi Arabia
- UAE
- South Africa
- Israel
- Egypt
- Rest of MEA
Competitive Landscape
The global companion diagnostic market is exceedingly dynamic and has the presence of diverse pharmaceutical companies, from large multinational groups to smaller nearby players. Many important pharmaceutical corporations which including Abbott Laboratories Inc. and Agilent Technologies Inc., are actively involved within the associate diagnostic market, as they are searching to develop partner diagnostics treatments to gain a competitive edge over different organizations in this marketplace.
Companies operating in this market frequently collaborate with diagnostic companies to co-broaden and commercialize these exams. For example, Roche, Pfizer, and Novartis are some of the most important pharmaceutical key market players inside the partner diagnostic marketplace who have collaborated with the diagnostic corporation to enhance their marketplace presence. Diagnostic businesses specialize in the development and commercialization of diagnostic exams, along with companion diagnostics.
They have know-how in numerous diagnostic technologies such as genomics and
in vitro diagnostics. Companies like Roche Diagnostics, Qiagen, and Agilent Technologies are outstanding in this section. Academic establishments and research organizations are also contributing to the development of the companion diagnostics marketplace, especially within the discovery of new biomarkers and technologies.
Some of the prominent players in the Global Companion Diagnostic Market are:
- Abbott Laboratories Inc.
- Agilent Technologies Inc.
- F.Hoffmann-La Roche Ltd
- Biomerieux SA
- Qiagen NV
- Siemens Healthcare
- Thermo Fisher Scientific Inc.
- Danaher Corporation
- Almac Group
- Illumina Inc.
- Myriad Genetics Inc.
- Guardant Health Inc.
- Biogenex Laboratories Inc
- Others
Recent Development
- In February 2024, F. Hoffmann-La Roche Ltd partnered with PathAI to develop AI-driven pathology algorithms, enhancing their companion diagnostics.
- In November 2023, Amoy Diagnostics Co., Ltd. collaborated with Cell Signaling Technology to advance precision oncology through companion diagnostics development in China.
- In August 2023, Agilent Technologies, Inc. obtained European IVDR Certification for their Companion Diagnostic Assay.
- In August 2023, QIAGEN received FDA approval for a companion diagnostic to assist in therapy selection for gastrointestinal stromal tumors alongside Blueprint Medicines’ AYVAKIT.
- In March 2023, F. Hoffmann-La Roche Ltd received FDA approval for expanding the label of VENTANA PD-L1 (SP263) Assay, aiding in identifying patients with specific types of lung cancer eligible for Libtayo.
- In September 2022, Thermo Fisher Scientific Inc. announced FDA Approval for the Oncomine Dx Target Test, the first NGS-based companion diagnostic for aiding therapy selection in patients with RET mutations/fusions in thyroid cancers.
Report Details
|
Report Characteristics
|
| Market Size (2024) |
USD 9.0 Bn |
| Forecast Value (2033) |
USD 26.0 Bn |
| CAGR (2024-2033) |
12.5% |
| Historical Data |
2018 – 2023 |
| Forecast Data |
2024 – 2033 |
| Base Year |
2023 |
| Estimate Year |
2024 |
| Report Coverage |
Market Revenue Estimation, Market Dynamics, Competitive Landscape, Growth Factors and etc. |
| Segments Covered |
By Product (Assay, Kits & Reagents and Software & Services), By Technology (Polymerase Chain Reaction (PCR), Real-time PCR (RT-PCR), In-situ Hybridization (ISH), Immunohistochemistry (IHC), Gene Sequencing and Others), By Indication (Cancer, Neurological disease, Infectious Disease and Other), By Sample Type (Tissue Samples, Blood Samples, and Others), By End User (Pharmaceutical & Biopharmaceutical Companies, Reference Laboratories, Contract Research Organizations, and Others) |
| Regional Coverage |
North America – The US and Canada; Europe – Germany, The UK, France, Russia, Spain, Italy, Benelux, Nordic, & Rest of Europe; Asia- Pacific– China, Japan, South Korea, India, ANZ, ASEAN, Rest of APAC; Latin America – Brazil, Mexico, Argentina, Colombia, Rest of Latin America; Middle East & Africa – Saudi Arabia, UAE, South Africa, Turkey, Egypt, Israel, & Rest of MEA |
| Prominent Players |
Abbott Laboratories Inc., Agilent Technologies Inc., F.Hoffmann-La Roche Ltd, Biomerieux SA, Qiagen NV, Siemens Healthcare, Thermo Fisher Scientific Inc., Danaher Corporation, Almac Group, Illumina Inc., Myriad Genetics Inc., Guardant Health Inc., Biogenex Laboratories Inc, and Other Key Players |
| Purchase Options |
HVMN Inc., Thync Global Inc., Apple Inc., Fitbit Inc., TrackmyStack, OsteoStrong, The ODIN, Thriveport LLC, Muse, Moodmetric, and Other Key Players |
Frequently Asked Questions
The Global Companion Diagnostics Market size was valued USD 8.0 billion in 2023 and is expected to reach a market value of USD 26.0 billion in 2033.
The Global Companion Diagnostics Market is anticipated to grow with a CAGR (Compound annual growth rate) of 12.5% during the forecast period.
North America is expected to dominate the Global Companion Diagnostics Market with 39.3% of the total revenue share in 2024.
Some of the prominent players Global Companion Diagnostics Market include Abbott Laboratories Inc.,Agilent Technologies Inc., F.Hoffmann-La Roche Ltd, and other key players.