Demand for ethical, environmentally sustainable testing methods has led to greater adoption of in vitro toxicology solutions across pharmaceutical and chemical industries. Global regulatory agencies are placing increased focus on eliminating animal testing while driving advances in alternative testing methodologies and expanding markets. Integration with
applications is also accelerating the adoption of these technologies, particularly in drug safety assessment and clinical trials.
The international in vitro toxicology marketplace refers to the collective industry involved inside the improvement, manufacturing, and commercialization of technology, products, and offerings associated with the assessment of toxicological effects of materials outdoor of living organisms.
In vitro toxicology entails accomplishing experiments in managed environments, which include check tubes or other artificial setups, to have a look at the doubtlessly toxic consequences of chemical compounds, drugs, and diverse substances on cells, tissues, and organs.
Asia-Pacific emerging markets are currently experiencing substantial growth due to increased investments in pharmaceutical and biotechnology research. Furthermore, as more companies shift toward personalized medicine solutions for treatment purposes, demand has grown for reliable in vitro testing methods at competitive costs that create opportunities in this market. This is particularly relevant for Cancer Immunotherapy and novel biopharmaceutical drugs requiring precise toxicity profiling.
Future market growth will be determined by innovations in AI and machine learning to advance predictive modeling of toxicity. Concerns over environmental and human healt
h risks have increased demand for faster, more accurate in-vitro testing solutions; sustainable innovation remains a driving force of market expansion.
In terms of geographical distribution, North America holds the largest market share, contributing around 40% of global revenue. The Asia-Pacific region is experiencing a rapid adoption rate, with a 15% increase in market growth annually. Regulatory pressures from agencies like the FDA and EU have further accelerated the market's transition toward safer, non-animal testing methodologies.
Key additives of the market consist of technology, assays, merchandise, and reagents utilized in toxicity testing, in addition to offerings in vitro toxicology studies. The market serves critical roles in drug discovery, ensuring safety before clinical trials, and addressing ethical issues related to traditional animal testing strategies. It plays a pivotal position in diverse industries, along with prescription drugs, cosmetics, chemicals, meals, and environmental testing.
Key Takeaways
- Market Size & Growth: The global in-vitro toxicology market is projected to reach USD 36.4 billion in 2023 and expand to USD 104.8 billion by 2032, growing at a robust CAGR of 12.5%, driven by the shift toward non-animal testing methods and rising regulatory requirements.
- Leading Technologies & Methods: Cell culture technology dominates the market with a 43.1% share in 2023, while cellular assays lead by method at 44.6%, due to their high physiological relevance, versatility, and suitability for high-throughput toxicity testing.
- Key Applications: Systemic toxicology accounts for 64.2% of the market share, reflecting its importance in evaluating comprehensive toxic effects on organisms, critical for drug development, chemical safety, and regulatory compliance.
- End Users: The pharmaceutical industry is the primary end-user segment with 45.1% market share, leveraging in-vitro toxicology for drug discovery, early toxicity assessment, and ethical testing practices; other sectors include cosmetics, chemicals, food, and diagnostics.
- Regional Performance: North America dominates the market with 49.4% of global revenue in 2023, supported by high healthcare expenditure, strong R&D infrastructure, stringent regulatory requirements, and the presence of key market players.
Use Cases
- Drug Safety Assessment: In-vitro toxicology is extensively used by pharmaceutical companies to evaluate the safety profile of drug candidates before clinical trials, reducing risks of adverse effects and improving regulatory compliance.
- Cosmetic & Consumer Product Testing: Cosmetic and household product manufacturers leverage in-vitro toxicology methods, including 3D skin models, to assess dermal toxicity and irritation, supporting ethical alternatives to animal testing.
- Environmental & Chemical Safety: Chemical industries utilize in-vitro toxicology to test the toxic effects of industrial chemicals and pollutants on human cell lines and tissues, aiding in environmental risk assessment and regulatory reporting.
- Personalized Medicine & Biologics: Patient-derived organoids and 3D cell cultures enable customized toxicity testing for tailored therapies, allowing pharmaceutical companies to optimize drug safety and efficacy for specific patient populations.
- High-Throughput Screening for Drug Discovery: Integration of AI and high-throughput screening (HTS) platforms allows rapid testing of thousands of compounds, accelerating the identification of toxic effects and streamlining the drug discovery pipeline.
Market Dynamic
The global in vitro toxicology marketplace is formed by several key factors. Ethical issues and regulatory tasks are riding a shift toward in vitro methods as options for animal testing. Stringent regulatory necessities, particularly in drug development, propel the adoption of reliable in vitro testing. Increasing worldwide healthcare expenditure supports investments in in vitro toxicology, even as packages amplify past prescribed drugs to industries like cosmetics, chemical substances, meals, and the surroundings.
Integration of omics technology enhances molecular insights, and the need for high throughput screening and automation is rising. Collaborations among industry gamers and studies corporations pressure advancements, however challenges in accomplishing whole predictive accuracy persist.
The marketplace has witnessed the entry of the latest players, fostering opposition and innovation. Understanding and navigating these changing dynamics are crucial for stakeholders inside the international vitro toxicology market to advantage a competitive aspect over others.
Driving Factors
An increasing emphasis on animal testing reduction drives the in-vitro toxicology market. Regulatory bodies, ethical considerations and public concerns all advocate for alternative means to assess toxicology. In-vitro models such as 3D cell cultures and organ-on-chip technologies provide effective, cost-efficient, and ethical testing solutions for pharmaceuticals, cosmetics, and chemicals.
Advancements in molecular biology and high-throughput screening methods have greatly increased the accuracy of in vitro toxicology models, encouraging their widespread adoption. Governments and organizations around the world also advocate for non-animal testing via funding or policy initiatives, thus driving market expansion for in vitro toxicology as an acceptable alternative.
Trending Factors
Artificial Intelligence (AI) and High-Throughput Screening (HTS) technologies are revolutionizing in-vitro toxicology testing. AI-powered algorithms enable rapid analysis of large datasets, improving accuracy in toxicity predictions while simultaneously screening thousands of compounds; HTS techniques expedite drug discovery processes.
Combining both technologies expedites identification of toxic effects earlier in development cycles while simultaneously cutting costs and time commitments for drug discovery projects. This trend has proven especially popular within pharmaceutical and Biopharmaceutical sectors due to their focus on efficiency, precision, and innovation for testing toxicity applications.
Restraining Factors
One major impediment to growth for in-vitro toxicology market is its inconsistency between industries in terms of testing protocols. Varying methodologies and result interpretations impede regulatory approval as well as cross-industry adoption. Consistency between data is an issue compromising its reliability and comparability, creating challenges when validating in-vitro models.
Transitioning away from traditional animal-based methods towards in vitro alternatives requires significant investments of infrastructure and expertise that may deter smaller companies. Addressing these challenges requires collaborative efforts among regulatory bodies, researchers, and industry stakeholders in the form of harmonized guidelines and protocols that ensure credibility and widespread use of in vitro toxicology methods.
Opportunity
The proliferation of personalized medicine provides significant opportunity for in-vitro toxicology companies. Tailor-made treatments require comprehensive toxicity profiling in order to guarantee patient safety and efficacy. In-vitro models such as patient-derived organoids and 3D cultures enable tailored toxicity assessments tailored to personalized therapies, meeting their demands.
With increased emphasis on targeted drug delivery systems and biologics, advanced in vitro testing methods become even more critical to meeting patient needs. Companies investing in innovative technologies or forming collaborations with precision medicine initiatives may capitalize on this lucrative opportunity to drive the market expansion while furthering advances in personalized healthcare solutions, including
Neurological Disorder Drugs and
Cancer Immunotherapy development.
Research Scope and Analysis
By Technology
Cell culture generation stands out as a dominant force inside the in vitro toxicology market in this segmentation based on generation as it
holds 43.1% of the market share in 2023 and is anticipated to show growth in the approaching duration of 2023 to 2032.
The dominance of cell culture is due to its unprecedented physiological relevance, versatility with numerous cellular types and three-D fashions, reproducibility, suitability for excessive throughput screening, and flexibility for long-term studies.
Ongoing technological advancements, integration with different contemporary technologies, and its potential to address ethical worries via reducing reliance on animal testing further contribute to its prominence. The complete insights into biological and molecular endpoints make cellular culture generation a cornerstone for in vitro toxicology studies, solidifying its position as a main and fundamental generation inside this market.
By Method
Cellular assays hold a dominant position in the in vitro toxicology market based on the method as they hold %44.6 of the market share in 2023 and are anticipated to show significant growth in the forthcoming period of 2023 to 2032.
Cellular assay dominates this segment as they can closely mimic biological systems, providing relevant information about how substances may behave in the human body. Their high throughput screening capability is crucial for efficiently assessing numerous compounds, especially in pharmaceutical research.
The diversity of cell types and models, functional readouts, and the ability to provide mechanistic insights contribute to their versatility. Technological advancements, such as imaging technologies, enhance their capabilities, and their application spans various industries beyond pharmaceuticals. Integration with automation and robotics streamlines processes and regulatory acceptance for certain assessments further solidifying the prominence of cellular assays in modern toxicology research and drug development.
By Application
Systemic toxicology holds a dominant position in the field of in vitro toxicology in the context of application as it holds 64.2% of the market share in 2023 and is anticipated to show subsequent growth in the upcoming period of 2023 to 2032. They dominate this segment as they involve a comprehensive assessment of toxic effects on the entire organism, providing crucial insights into the safety profile of substances, especially in pharmaceutical drug development.
This method is incredibly relevant to human health, as it examines how materials affect exceptional organs and physiological structures. Regulatory compliance, mainly with businesses like the FDA and EMA, mandates thorough exams of systemic toxicity. The identification of organ-specific effects, predictive modeling for human responses, and collaboration across disciplines make systemic toxicology robust and informative.
Its importance extends beyond pharmaceuticals to assess risks associated with environmental and industrial chemicals. In essence, the dominance of systemic toxicology in the in vitro setting reflects its pivotal role in understanding the complex, organism-wide effects of substances, essential for informed decision-making across diverse industries.
By End User
In vitro toxicology performs a vital role in drug discovery by assessing the safety and toxicity of ability drug applicants, aligning with the enterprise's consciousness on early identification of capability issues. Regulatory compliance is a key motive force, as in vitro strategies enable thorough protection checks in step with stringent requirements, lowering the risk of destructive consequences in later medical ranges. The enterprise's commitment to ethical practices, along with the discount of animal checking out, aligns with the ethical concerns related to in vitro toxicology.
Furthermore, the pharmaceutical area values the cost and time performance of in vitro assays, presenting excessive-throughput screening and early difficulty identity, supporting the industry's want for expanded drug improvement and decreased prices. The rise of customized medicinal drugs and focused treatment plans has further fueled the enterprise's reliance on in vitro toxicology, permitting the evaluation of drug responses in precise patient populations.
The In-Vitro Toxicology Market Report is segmented on the basis of the following
By Technology
- Cell Culture Technology
- High Throughput Technology
- Molecular Imaging
- Omics Technology
By Method
- Cellular Assay
- Biochemical Assay
- In-silico
- Ex-vivo
By Application
- Systemic Toxicology
- Dermal Toxicity
- Endocrine Disruption
- Ocular Toxicity
- Others
By End User
- Pharmaceutical Industry
- Cosmetic & Household Products
- Academic Institutes & Research Laboratories
- Diagnostics
- Chemical Industry
- Food Industry
Regional Analysis
The global in vitro toxicology market is led by North America, which has
49.4% of the market share as of 2023 and it is estimated to show growth during the forecast period from 2023 to 2032. The region benefits from a robust studies and development infrastructure, with leading pharmaceutical and biotechnology businesses, and educational establishments, riding innovation and creating the need for advanced in vitro toxicology solutions. High healthcare expenditure in the United States fuels the adoption of contemporary technologies for drug discovery and improvement.
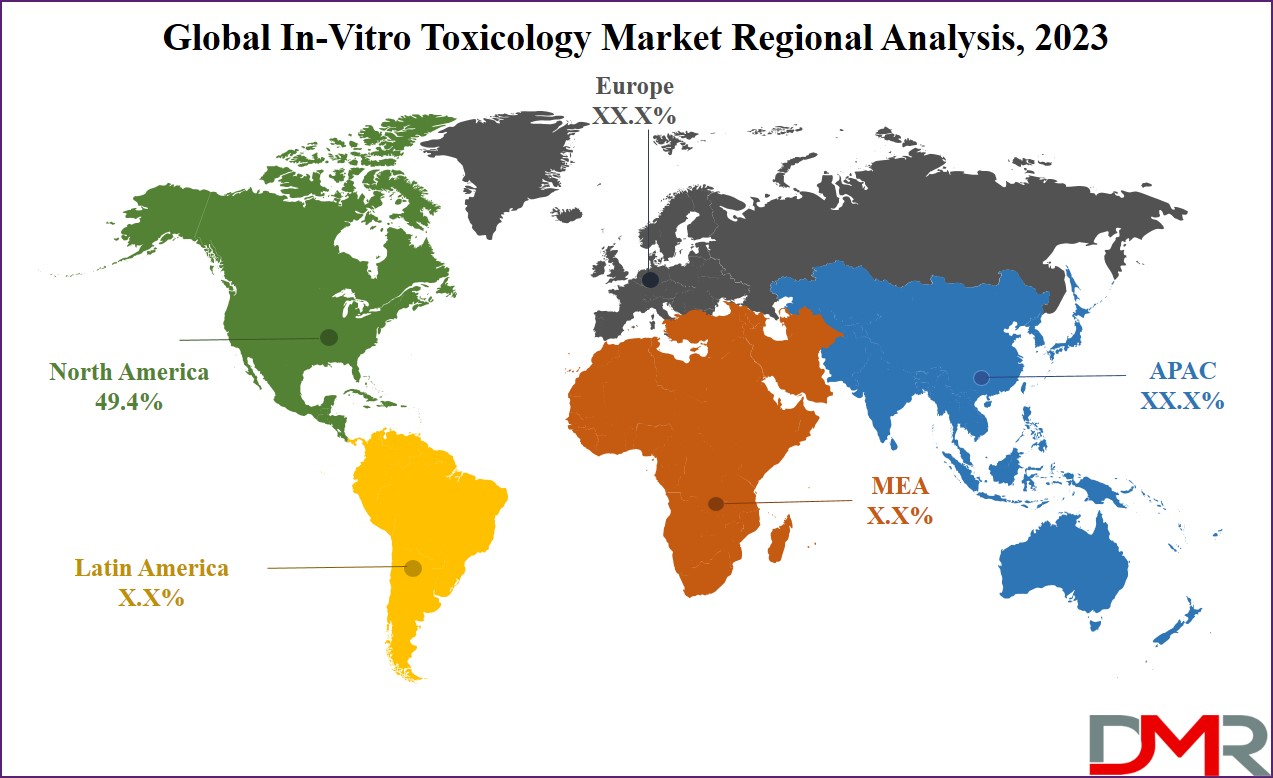
The stringent regulatory surroundings, in particular, enforced by way of the FDA, motivate corporations to spend money on advanced in vitro toxicology techniques to satisfy regulatory standards efficaciously. The presence of key marketplace gamers, consisting of Thermo Fisher Scientific and Charles River Laboratories, established or substantially based totally in North America, further contributes to the vicinity's dominance.
Collaborations and partnerships within the area foster technological improvements, whilst a properly knowledgeable market and robust focus on protection testing blessings support the substantial adoption of in vitro toxicology methods.
By Region
North America
Europe
- Germany
- The U.K.
- France
- Italy
- Russia
- Spain
- Benelux
- Nordic
- Rest of Europe
Asia-Pacific
- China
- Japan
- South Korea
- India
- ANZ
- ASEAN
- Rest of Asia-Pacific
Latin America
- Brazil
- Mexico
- Argentina
- Colombia
- Rest of Latin America
Middle East & Africa
- Saudi Arabia
- UAE
- South Africa
- Israel
- Egypt
- Rest of MEA
Competitive Landscape
The worldwide in vitro toxicology marketplace is characterized by an aggressive landscape presenting prominent gamers including Thermo Fisher Scientific, Merck KGaA (Sigma Aldrich), Charles River Laboratories International, Covance Inc. (LabCorp), and Agilent Technologies.
These groups provide a wide variety of services and products to satisfy the growing demand for green and value-effective toxicology testing methods. The marketplace competitiveness is motivated by non-stop technological improvements in mobile subculture technologies, 3-D cell culture models, and excessive-throughput screening techniques.
Strategic collaborations and partnerships with academic institutions, studies agencies, and industry friends contribute to enhancing product offerings and increasing market reach. Regulatory compliance is a critical aspect, and organizations adhering to stringent requirements benefit from an aggressive gain. Market enlargement, both geographically and via research and development tasks, is a not unusual method amongst key players.
Some of the prominent players in the Global In-Vitro Toxicology Market are
- Charles River Laboratories International, Inc.
- Merck KGaA
- Eurofins Scientific
- Abbott Laboratories
- Laboratory Corporation of America Holdings
- Evotec S.E.
- Thermo Fisher Scientific Inc.
- Quest Diagnostics Incorporated
- Agilent Technolgies Inc.
- Catalent Inc.
- Danaher Corporation
- Bio-Rad Laboratories Inc.
- Other Key Players
Recent Developments
- In March 2023, Agilent Technologies Inc. Acquired e-MSion via acquisition. In this acquisition, Agilent will combine the e-MSion’s ExD cell into its portfolio of advanced workflows, instruments, devices, and analytical solutions for biotherapeutic characterization and improvement.
- In January 2023, Eurofins Scientific accelerated its presence in India with the status quo of a brand new, completely geared up, modern-day laboratory campus in Genome Valley, Hyderabad. The lab will assist pharma and biotech businesses inside the regions of synthetic natural chemistry, analytical research, and improvement, bioanalytical services, in vivo pharmacology, safety toxicology, and system research and improvement.
- In September 2022, BioIVT proudly introduced the crowning glory of its acquisition of XenoTech, underscoring its commitment to providing comprehensive studies fashions and services to biopharmaceutical customers for advancing drug development and diagnostic research.
Report Details
| Report Characteristics |
| Market Size (2023) |
USD 36.4 Bn |
| Forecast Value (2032) |
USD 104.8 Bn |
| CAGR (2023-2032) |
12.5% |
| Historical Data |
2018 – 2023 |
| Forecast Data |
2024 – 2033 |
| Base Year |
2023 |
| Estimate Year |
2024 |
| Report Coverage |
Market Revenue Estimation, Market Dynamics, Competitive Landscape, Growth Factors and etc. |
| Segments Covered |
By Technology (Cell Culture Technology, High Throughput Technology, Molecular Imaging, and Omics Technology), By Method (Cellular Assay, Biochemical Assay, In-silico, and Ex-vivo), By Application (Systemic Toxicology, Dermal Toxicity, Endocrine Disruption, Ocular Toxicity, and Others), By End User (Pharmaceutical Industry, Cosmetic & Household Products, Academic Institutes & Research Laboratories, Diagnostics, Chemical Industry, and Food Industry) |
| Regional Coverage |
North America – The US and Canada; Europe – Germany, The UK, France, Russia, Spain, Italy, Benelux, Nordic, & Rest of Europe; Asia- Pacific– China, Japan, South Korea, India, ANZ, ASEAN, Rest of APAC; Latin America – Brazil, Mexico, Argentina, Colombia, Rest of Latin America; Middle East & Africa – Saudi Arabia, UAE, South Africa, Turkey, Egypt, Israel, & Rest of MEA |
| Prominent Players |
Charles River Laboratories International Inc., Merck KGaA, Eurofins Scientific, Abbott Laboratories, Laboratory Corporation of America Holdings, Evotec S.E., Thermo Fisher Scientific Inc., Quest Diagnostics Incorporated, Agilent Technolgies Inc., Catalent Inc., Danaher Corporation, Bio-Rad Laboratories Inc., and Other Key Players |
| Purchase Options |
We have three licenses to opt for: Single User License (Limited to 1 user), Multi-User License (Up to 5 Users), and Corporate Use License (Unlimited User) along with free report customization equivalent to 0 analyst working days, 3 analysts working days and 5 analysts working days respectively. |